Aeromonas Hydrophila is a gram-negative bacterium which exists ubiquitously in water ponds. It is an opportunistic pathogen which can cause many diseases in fish and create a large amount of economic loss in aquaculture. The symptoms of the diseases caused by A. hydrophila infection include ulcers, fin rot, and hemorrhagic septicaemia. When A. hydrophila enters into fish body, it often colonizes the gastrointestinal tract first. The virulence factors of A. hydrophila include its enterotoxins, hemolysin, and proteases produced by Type II and Type III secretion system. (Citterio) Current treatment and prevention of A. hydrophila infection include the use of antibiotics and vaccine. However, the pathogen has developed resistance to most of the common antibiotics, and the approach of A. hydrophila vaccine is still not fully developed. Moreover, when we visited the fish farming industry, the manager told us that it was difficult to identify A. hydrophila infection. (Vivekanandhan) Therefore, the goal of our 2018 iGEM project is to develop an A. hydrophila killer which can report potential infection to fish farming industry managers, reduce the virulence of A. hydrophila and kill the pathogen directly. Bacteria, both Gram-negative and Gram-positive, use quorum sensing (QS) to regulate their gene expression. QS pathways of Gram-negative bacteria contain two vital proteins analogous to LuxI and LuxR gene regulation. The LuxI-like protein is responsible for the synthesis of an autoinducer, lactone signaling molecules (HSL). The greater the bacteria-population density is, the greater the concentration of that autoinducer will be. After the concentration of an autoinducer accumulates to a certain threshold concentration, autoinducers and LuxR-like protein, a transcriptional regulator, bind together to form LuxR-autoinducer complexes which then activate the transcription of the target gene (Garde et al., 2010). The QS mechanism allows bacteria to regulate their gene expression efficiently based on their population density. As a Gram-negative bacillus, A. hydrophila uses AhyRI system, an analogous system to LuxRI system, to regulate gene expression(Miller & Bassler, 2001). The signal molecule C4-HSL, which takes the largest proportion among all signal molecules of A. hydrophila (Lynch et al., 2002), serves as autoinducer in its QS system. In an A. hydrophila population, C4-HSL is produced by AhyI. After the concentration of C4-HSL reaches a certain level, autoinducer binds to htranscriptional regulator AhyR, and the AhyR-C4-HSL complex, in turn, binds to the promoter region to activate transcription. Instead of using AhyR, however, we use RhlR as our transcriptional regulator. The RhlR-C4-HSL complex will bind to the promoter region and activate the transcription of the downstream gene in our engineered E. coli. Many pathogenesis-related factors of A. hydrophila, including expression of virulence factors (such as hemolysin, protease, S layer proteins, DNase, amylase), biofilm maturation, and the type II, III and VI secretion system, are reported under the regulation of quorum sensing in Aeromonas hydrophila. (Cao et al., 2012) In this project, we aim at cutting the QS pathway within A. hydrophila population to cure its infection on fish. To block QS within A. hydrophila, we engineer E. coli to make it express an autoinducer inactivation enzyme aiiA: after the transcriptional regulator RhlR is bound with C4-HSL in the environment, the complex activates the expression of aiiA that blocks the surrounding C4-HSL to reduce QS within A. hydrophila population. aiiA is a lactonase which cleaves the homoserine lactone ring of molecule AHLs in a hydrolytic and reversible reaction. The aiiA we use is AHL-lactonase147-11516 from Bacillus thuringiensis strain 147-11516(BBa_K2548004). It prefers AHLs with shorter acyl chains and is thus observed to have a higher specificity with C4-HSL. As it still presents the biological activity under alkaline pH conditions, it is effective in fish’s intestine. (Pedroza, Flórez, Ruiz & Orduz, 2013) We optimize the codons in the sequence BBa_C0160 as the second quorum quenching enzyme(BBa_K2548005). Antimicrobial peptides (AMPs) are proteins that protect the host species from microbial invasion. They are part of the innate immune system and are found among all classes of life. They kill microbes through depolarizing microbial membranes, scrambling the usual distribution of lipids and damaging crucial intracellular organelles. There are many kinds of antimicrobial peptides, and we use three specific AMPs to treat the invasion of A. hydrophila : Scygonadin, SpALF4, and cPcAMP1. (Huang) Scygonadin is an anionic antimicrobial peptide identified from the seminal plasma of the species Scylla serrata (Giant mud crab). It has shown the antimicrobial activity against A. hydrophila. Its DNA reveals that it is only expressed in male crabs, helping them to fight off bacterial invasions. However, it is difficult to transfer large amount of scygonadin from Scylla serrata to other organisms, so our plan is to produce peptide syngonadin in E. coli through genetic modification. Our aim is to insert the cDNA sequence coding for mature peptide of scygonadin into the genetic expression of E. coli. (Huang) Anti-lipopolysaccharide factors (ALFs) are antimicrobial peptides with a broad spectrum of antimicrobial activity. SpALF4 is an ALF homolog identified from Scylla paramamosain (Mud crab) that has shown antimicrobial activity against A. hydrophila. Our aim is to obtain the cDNA sequence of SpALF4 from Scylla paramamosain and insert it into the genetic expression of E. coli to express large amount of SpALF4 to treat A. hydrophila invasions. (Zhu) As an antimicrobial peptide that is not homologous to any AMPs currently known, cPcAMP1 is identified from Paramecium Caudatum and is able to kill A. hydrophila. The cPcAMP1 has the capacity to increase the permeability of bacterial cell membrane and depolarize bacterial cells. Furthermore, the cPcAMP1 is not toxic to eukaryotic cells; so, when it is applied to the fishes, it causes no harm. (Cui) Aeromonas Bacteria. (n.d.). Retrieved from www.medical-labs.net/aeromonas-bacteria- 2440/ Cao, Y., He, S., Zhou, Z., Zhang, M., Mao, W., Zhang, H., & Yao, B. (2012). Orally Administered ThermostableN-Acyl Homoserine Lactonase from Bacillus sp. Strain AI96 Attenuates Aeromonas hydrophila Infection in Zebrafish. Applied And Environmental Microbiology, 78(6), 1899-1908. doi: 10.1128/aem.06139-11 Citterio, B., & Biavasco, F. (2015). Aeromonas hydrophila virulence. Virulence, 6(5), 417-418. doi:10.1080/21505594.2015.1058479 Cui, P., Dong, Y., Li, Z., Zhang, Y., & Zhang, S. (2016). Identification and functional characterization of an uncharacterized antimicrobial peptide from a ciliate Paramecium caudatum. Developmental & Comparative Immunology,60, 53-65. doi:10.1016/j.dci.2016.02.016 Garde, C., Bjarnsholt, T., Givskov, M., Jakobsen, T. H., Hentzer, M., Claussen, A., . . . Sams, T. (2010). Quorum sensing regulation in Aeromonas hydrophila. J Mol Biol, 396(4), 849-857. doi:10.1016/j.jmb.2010.01.002 Huang, W. S., Wang, K. J., Yang, M., Cai, J. J., Li, S. J., & Wang, G. Z. (2006). Purification and part characterization of a novel antibacterial protein Scygonadin, isolated from the seminal plasma of mud crab, Scylla serrata (Forskål, 1775). Journal of Experimental Marine Biology and Ecology, 339(1), 37-42. doi:10.1016/j.jembe.2006.06.029 Lynch, M., Swift, S., Kirke, D., Keevil, C., Dodd, C., & Williams, P. (2002). The regulation of biofilm development by quorum sensing in Aeromonas hydrophila. Environmental Microbiology, 4(1), 18-28. doi: 10.1046/j. 1462-2920.2002.00264.x Miller, M. B., & Bassler, B. L. (2001). Quorum sensing in bacteria. Annu Rev Microbiol, 55, 165-199. doi:10.1146/annurev.micro.55.1.165 Pedroza, C., Flórez, A., Ruiz, O., & Orduz, S. (2013). Enzymatic hydrolysis of molecules associated with bacterial quorum sensing using an acyl homoserine lactonase from a novel Bacillus thuringiensis strain. Antonie Van Leeuwenhoek, 105(1), 253-264. doi: 10.1007/s10482-013-0072-5 Vivekanandhan, G., Savithamani, K., Hatha, A., & Lakshmanaperumalsamy, P. (2002). Antibiotic resistance of Aeromonas hydrophila isolated from marketed fish and prawn of South India. International Journal of Food Microbiology, 76(1-2), 165-168. doi:10.1016/s0168-1605(02)00009-0 Zhu, L., Lan, J., Huang, Y., Zhang, C., Zhou, J., Fang, W., Li, X. (2014). SpALF4: A newly identified anti-lipopolysaccharide factor from the mud crab Scylla paramamosain with broad spectrum antimicrobial activity. Fish & Shellfish Immunology,36(1), 172-180. doi:10.1016/j.fsi.2013.10.023Description
I. Aeromonas Hydrophila

Fig. 1 Aeromonas Hydrophila Infection (Aeromonas Bacteria)II. Quorum Sensing and Quorum Quenching
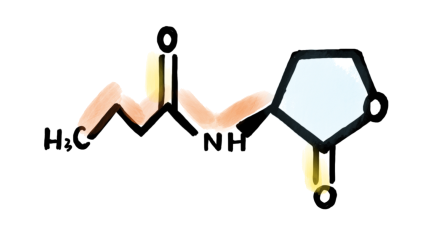
Fig 2. Molecular structure of C4-HSL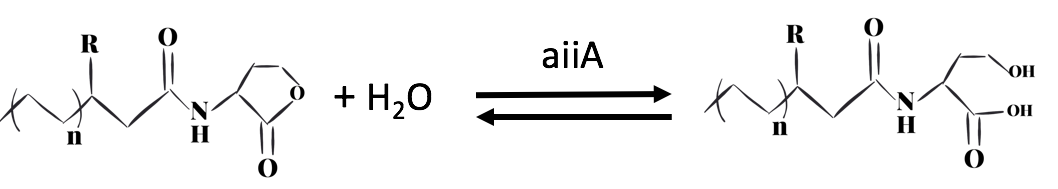
Fig 3. Inactivation of C4-HSL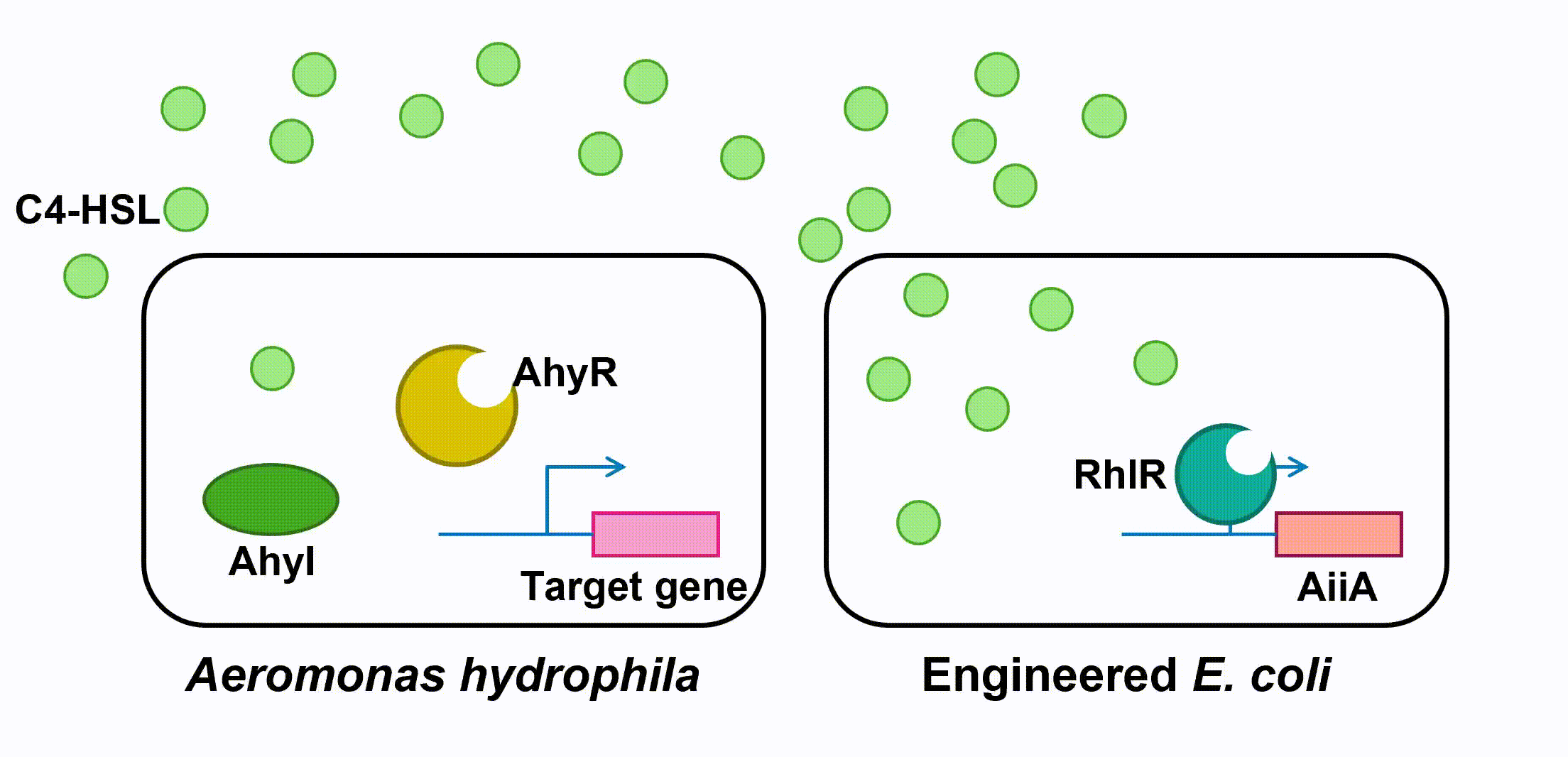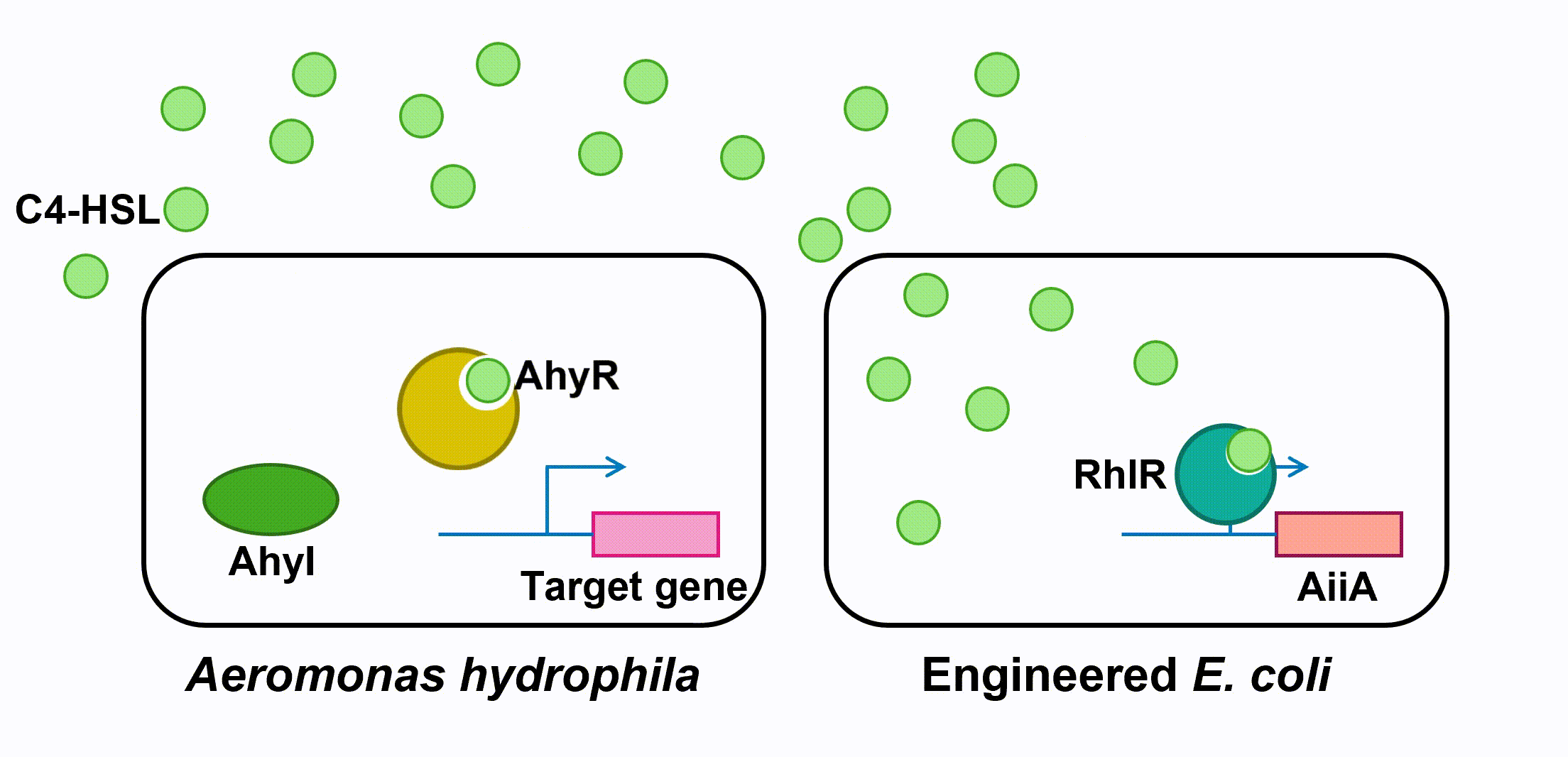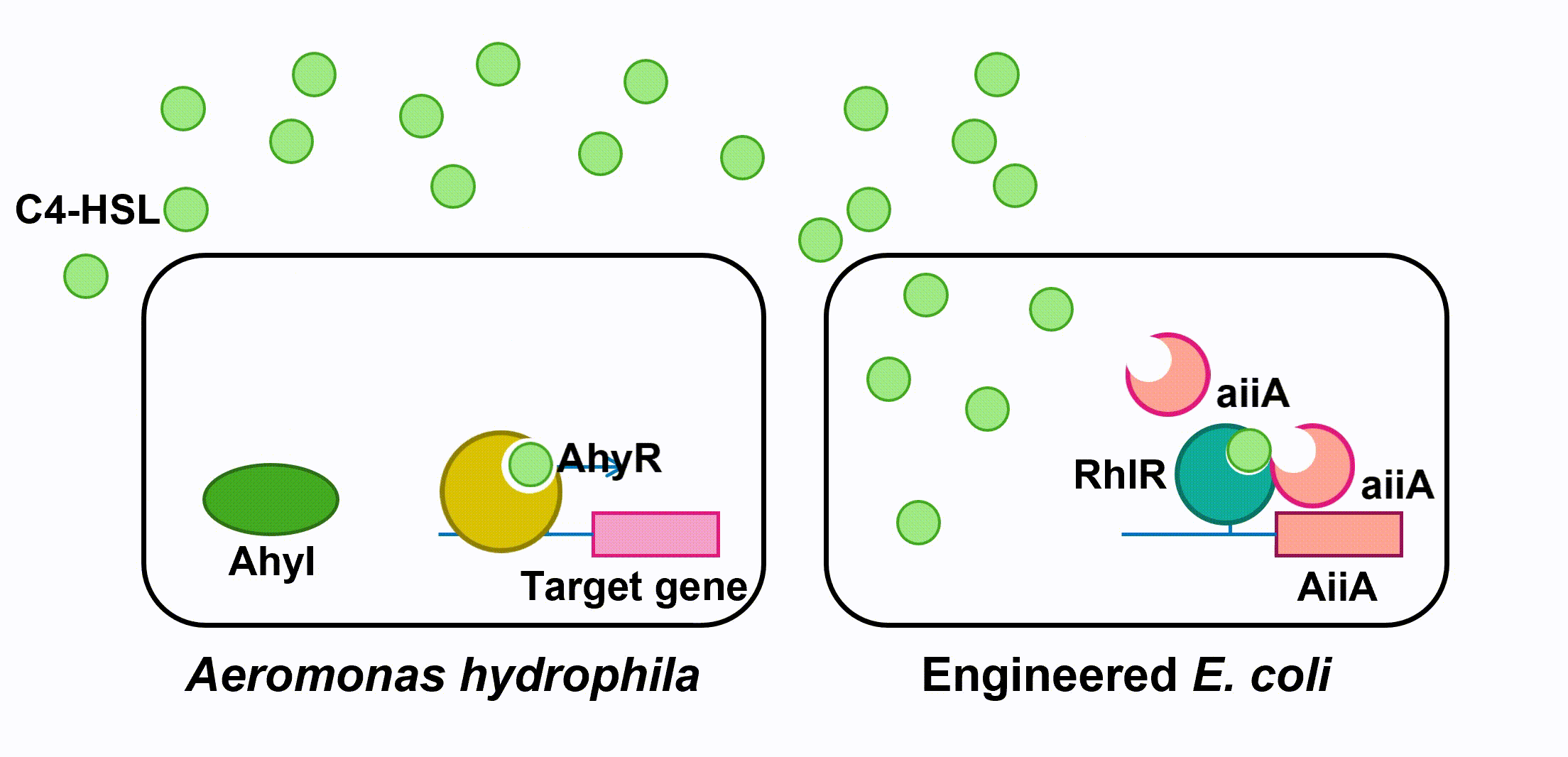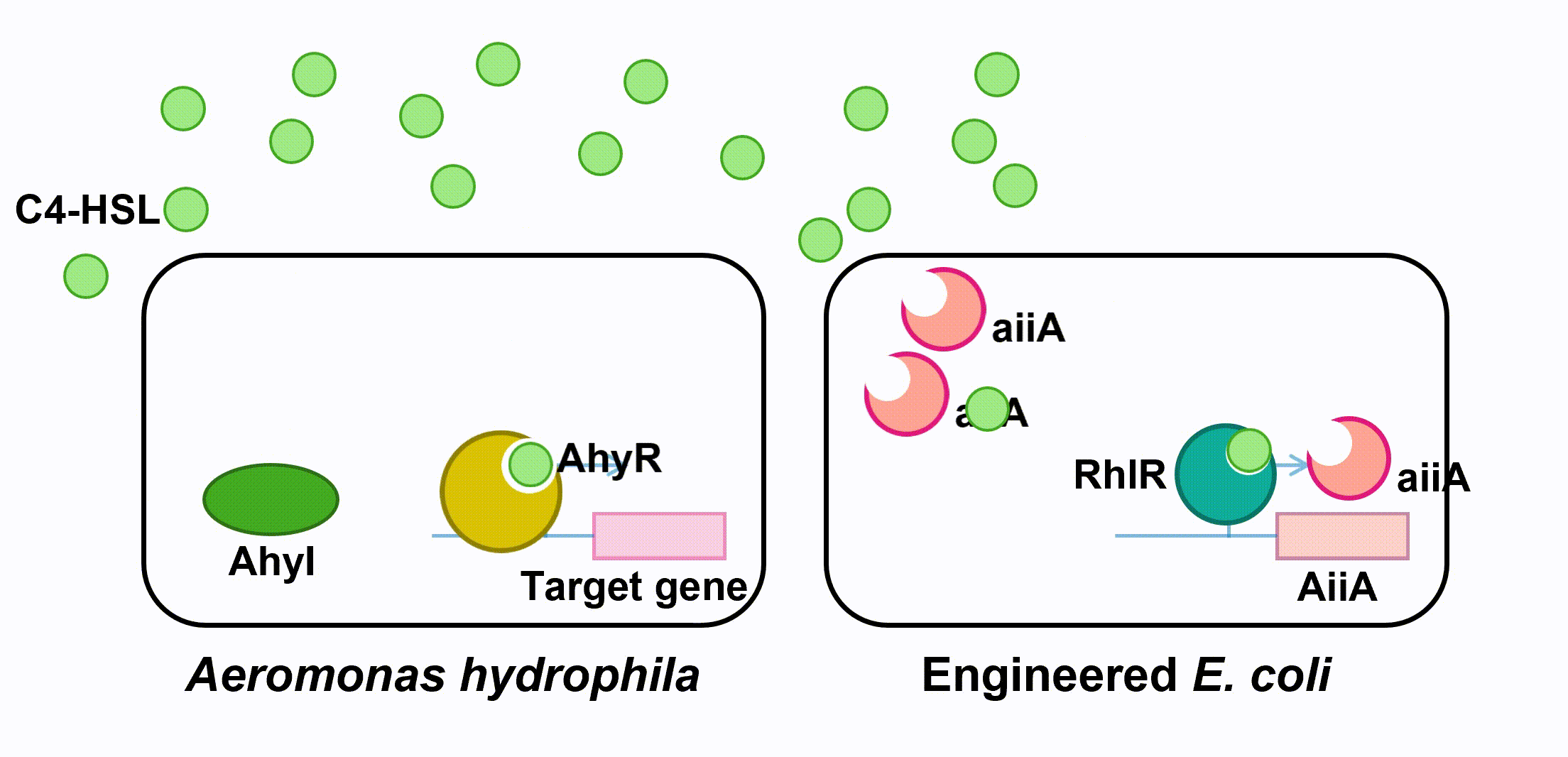
Fig 4. Quorum sensing of AhyRI and RhlRI system and Expression of aiiAIII. Antimicrobial Peptides

Fig 5. Effects of Antimicrobial peptides
References
