Background
Hyperuricemia refers to the abnormally high level of uric acid in the blood. And it’s closely associated with gout due to the deposition of monosodium urate crystals in peripheral joints and soft tissues, which will also cause acute gouty arthritis, chronic gouty arthritis and joint deformities.
This following table shows the incidence of hyperuricemia worldwide in 2015. As we can see the proportion is large in all area. With time going the proportion of hyperuricemia is gradually increasing. Although patients with hyperuricemia don’t have any obvious symptoms at the beginning. But as time goes by, various complications come out.

Table 1 Incidence of hyperuricemia worldwide in 2015
Hyperuricemia not only cause acute gouty arthritis but also damage the kidneys, causing abnormal kidney function, kidney stones, and other chronic kidney diseases. Besides it is also closely related to metabolic diseases such as hypertension, diabetes, heart diseases, and fatty liver.

Figure 1 Damage caused by hyperuricemia
The body's uric acid is mainly derived from the degradation of purine. At present, there are three types of drugs for hyperuricemia. The first one is to inhibit uric acid synthesis, mainly Febuxostat and Allopurinol; the second is to promote uric acid excretion, mainly Benzobromarone and Probenecid; the last one is to reduce acute gouty arthritis, mainly colchicine. However, these medicine have strong side effects. Allopurinol can cause gastrointestinal irritation, skin rashes, bone marrow suppression or liver damage. Benzobium Malone can cause allergic dermatitis, fever, gastrointestinal reaction, induced gout acute attack and colchicine also can cause severe diarrhea. So to develop a drug for hyperuricemia with less side effect is the focus of the world.

Figure 2 Synthetic pathways of uric acid and major therapeutic drugs
New weapon -- Urate Oxidase
In this section ,we are going to talk about our design ideas of the project. So here comes the problem.Is there a medicine that has good efficacy and has few side effect ? Of course,there is.That is the protagonist of our project today,the Urate Oxidase . .
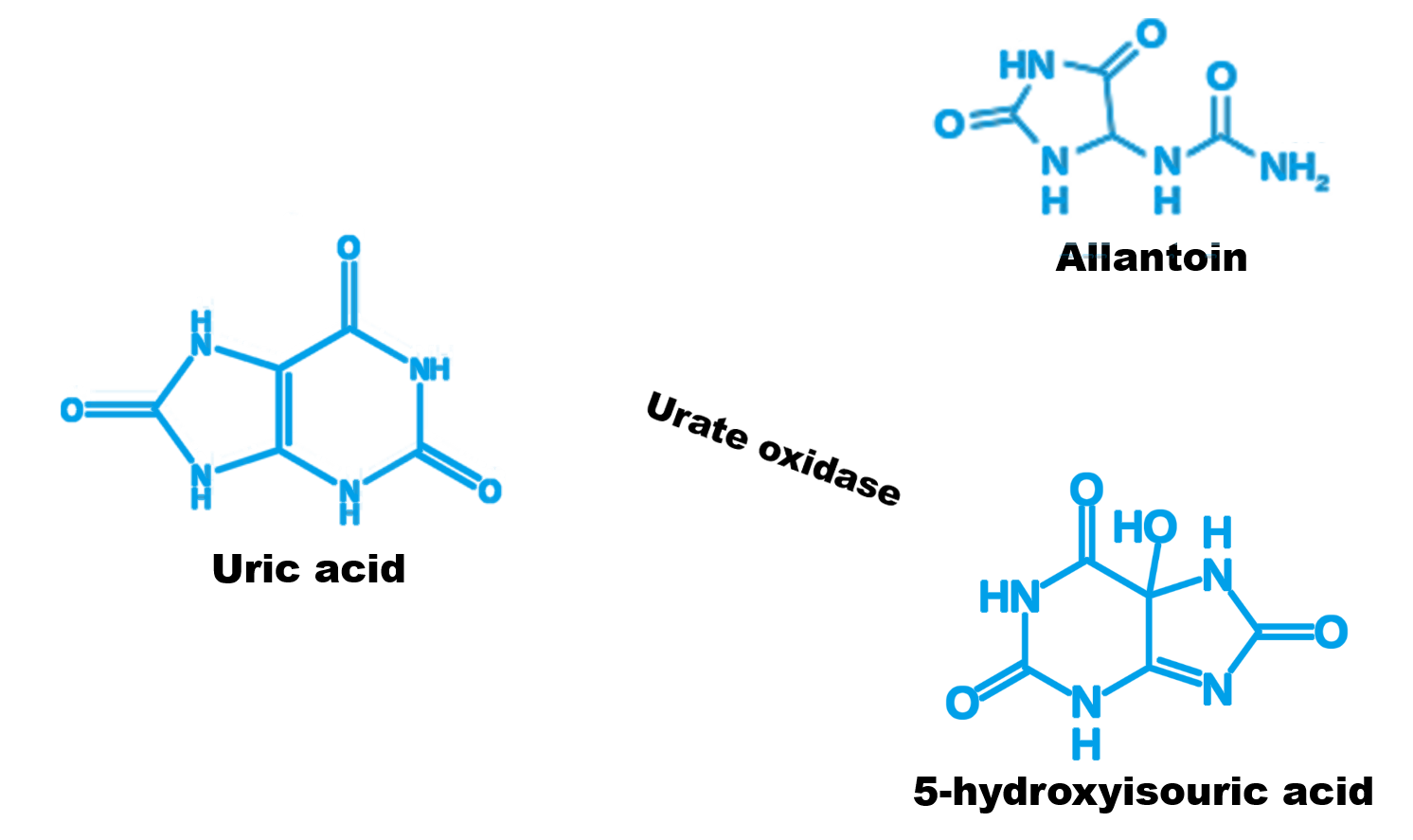
Figure 3 The reaction process of uric acid oxidase
Urate oxidase is an enzyme that catalyzes the oxidation of uric acid in purine metabolism .It oxidizes uric acid to 5-hydroxyisouric acid, which is a less stable compound and easily converted to allantoin .Allantoin can be easily metabolized by the kidneys, thereby reducing the uric acid level in the blood.So urate oxidase has been gradually recognized as a new type of therapeutic drug.
Urate oxidase is ubiquitous in living organisms. It can be synthesized by mold, mammals or insect . However, higher primates, such as orangutans, or humans, cannot synthesize urate oxidase. But why? Because the gene encoding urate oxidase in our body have a nonsense mutation , so we are unable to properly transcribe and translate this enzyme.

Our work

Our plan is to introduce the gene sequence encoding urate oxidase into Nissle E. coli and introduce these microorganisms into some drinks, such as yogurt.
When patients take these drinks ,part of the Nissle E. coli in the drinks will colonize the patient's intestines and when the uric acid concentration reaches a threshold ,the strain will secrete the urate oxidase with cell penetrating peptides , by this way ,it can respond to uric acid concentration in a long period of time. And moreover, the method does not need to send the medicine to the blood by injection, thereby reducing the pain of the patient during the treatment and is also more convenient.
In order to achieve this goal. we added a hypothetical uricase regulator, that is HucR, which is a transcriptional regulator a bacterium, this protein can interact with the consensus sequence of promotor for fricassee and promotor for HucR .we call the consensus sequence hucO.When HucR bind hucO, it will prevent RNA polymerase from binding to the promoter, thereby inhibiting the expression of related genes.
However,When uric acid molecules are present, uric acid can bind to HucR, making HucR unable to bind to hucO, and related genes can normally transcribe and translate.

We first plan to use RFP as a reporter gene to verify the feasibility of our system. When uric acid is absent or the concentration is not high, RFP is not expressed; but when uric acid concentration is sufficient, RFP is expressed and a red fluorescence can be seen.

In order to achieve drug penetration through the intestinal wall to the blood, we used cell signal peptides and cell penetrating peptides. The signal peptide is to make nissle icily secrete urate oxidase, and the penetrating peptide is to allow the enzyme to penetrate the intestinal wall cells to reach the blood. so, we selected 4 signal peptides and four penetrating peptides, as shown in the figure.

For more informaiton, please see our





