FMCommmichau (Talk | contribs) |
FMCommmichau (Talk | contribs) |
||
| Line 45: | Line 45: | ||
<p> | <p> | ||
<h4>Other applications for glyphosate</h4> | <h4>Other applications for glyphosate</h4> | ||
| − | <p>Companies like <a href="https://monsanto.com">Monsanto</a> with patented chemical technologies will generally try to patent all reasonable potential uses of that chemical in order to obtain maximal return on their research investments. Since glyphosate inhibits the aromatic biosynthetic pathway in many bacteria and parasites, and a reasonable case can be made that glyphosate might be effective as an antimicrobial. Indeed, <a href="https://monsanto.com">Monsanto</a> has filed in 2003 for the invention that the herbicidal agent glyphosate can be used in combination with the polyvalent anion oxalic acid to prevent and treat pathogenic infections caused by protozoan parasites of the phylum Apicomplexa (<a href="https://patentimages.storage.googleapis.com/86/6d/8e/2d98b85f6574ef/US7771736.pdf">Patent No.: US7,771,736B2</a>). However, a lot stands between glyphosate as a substance with antimicrobial activity in the test tube and a clinically effective antimicrobial agent. A reliable effective concentration of glyphosate with a reasonable oral dose in humans is difficult to achieve, especially when glyphosate is orally applied. Moreover, glyphosate only shortly persists in humans. Glyphosate blocks the production of <i>de novo</i> synthesis of aromatic amino acids in bacteria, and the bacteria will die, or at least stop reproducing, if they cannot obtain these nutrients from the environment … but blood and tissues are not water - they are chock-full of the nutrients that microbes need to survive. The addition of aromatic amino acids to the growth medium of bacteria indeed interferes with glyphosate-dependent inhibition of the EPSP synthase (6). <b>Moreover, nobody has demonstrated so far that glyphosate is an effective antimicrobial agent for treating human or animal infections</b>. | + | <p>Companies like <a href="https://monsanto.com">Monsanto</a> with patented chemical technologies will generally try to patent all reasonable potential uses of that chemical in order to obtain maximal return on their research investments. Since glyphosate inhibits the aromatic biosynthetic pathway in many bacteria and parasites, and a reasonable case can be made that glyphosate might be effective as an antimicrobial. Indeed, <a href="https://monsanto.com">Monsanto</a> has filed in 2003 for the invention that the herbicidal agent glyphosate can be used in combination with the polyvalent anion oxalic acid to prevent and treat pathogenic infections caused by protozoan parasites of the phylum Apicomplexa (<a href="https://patentimages.storage.googleapis.com/86/6d/8e/2d98b85f6574ef/US7771736.pdf">Patent No.: US7,771,736B2</a>). However, a lot stands between glyphosate as a substance with antimicrobial activity in the test tube and a clinically effective antimicrobial agent. A reliable effective concentration of glyphosate with a reasonable oral dose in humans is difficult to achieve, especially when glyphosate is orally applied. Moreover, glyphosate only shortly persists in humans. Glyphosate blocks the production of <i>de novo</i> synthesis of aromatic amino acids in bacteria, and the bacteria will die, or at least stop reproducing, if they cannot obtain these nutrients from the environment … but blood and tissues are not water - they are chock-full of the nutrients that microbes need to survive. The addition of aromatic amino acids to the growth medium of bacteria indeed interferes with glyphosate-dependent inhibition of the EPSP synthase (6). <b>Moreover, nobody has demonstrated so far that glyphosate is an effective antimicrobial agent for treating human or animal infections</b>. Therefore, it remains to be determined whether glyphosate may indeed act as an efficient and reliable antimicrobial agent to fight pathogenic bacteria and protozoan parasites. |
</p> | </p> | ||
| Line 52: | Line 52: | ||
<p> | <p> | ||
<h4>The glyphosate controversy</h4> | <h4>The glyphosate controversy</h4> | ||
| + | <p>Glyphosate is | ||
| + | |||
| + | <p> | ||
| + | <h4>The pros & cons of glyphosate usage in agriculture</h4> | ||
<p>Glyphosate is | <p>Glyphosate is | ||
Revision as of 07:47, 12 September 2018
Team Göttingen
iGEM 2018

Glyphosate on my plate?

Background
The discovery of the weedkiller glyphosate
Glyphosate (N-(phosphonomethyl)glycine) was first synthesized by the chemist Dr. Henri Martin in 1950, while working for the Swiss pharmaceutical company Cilag, which was founded in 1936 in Schaffhausen. Unfortunately (fortunately?), Dr. Martin did find out that glyphosate may serve as a very efficient herbicide (see below). About 20 years later, the American chemist Dr. John E. Franz who was working for the American company Monsanto (recently bought by Bayer) observed that glyphosate specifically inhibits the 5-enolpyruvyl-shikimate-3-phosphate (EPSP) synthase in plants, fungi, bacteria and archaea (Figure 1) (1-4). The EPSP synthase generates the precursor for the de novo synthesis of aromatic amino acids tryptophan, tyrosine and phenylalanine (5). Therefore, inhibition of the EPSP synthase by glyphosate results in the depletion of the cellular levels of aromatic amino acids and death of the organism that is treated with the weedkiller (Figure 1) (1,6-8).

Figure 1. Glyphosate that is present in Roundup specifically inhibits the 5-enolpyruvyl-shikimate-3-phosphate (EPSP) synthase (PDBid: 2QFU), which converts shipmate-3-phosphate (S3P) and phosphoenolpyruvate (PEP) to EPSP. A drop in the cellular levels of the precursor for aromatic amino acid synthesis causes death of the organism that has been treated with Roundup.
The usage of glyphosate
Glyphosate is resistant to chemical hydrolysis, thermal decomposition and photolysis due to a stable C-P bond (9). Moreover, due to the fact that glyphosate is toxicologically safe and that transgenic, glyphosate-resistant crops have been introduced by Monsanto, the herbicide has become the dominant weedkiller worldwide (10-15). The production and usage rate of Glyphosate increasing up to this day (Figure 2). Moreover, given the fact that genetically modified crops that produce glyphosate-insensitive EPSP synthases are tolerated in the united states, glyphosate is the most used herbicide in this country (13).

Figure 2. Glyphosate usage over the last two decades. It has been estimated that the production of glyphosate is increasing with a rate of about 40 tons per year. Adapted from Benbrook (13).
The problem of glyphosate-resistant crops
Due to the intensive application of glyphosate in agriculture, several plant species have become resistant against the herbicide. Please check out the webpage http://www.weedscience.org, which is reporting of herbicide resistant weeds globally. The international survey of herbicide resistant weeds is a collaborative effort between weed scientists in over 80 countries. The main aim of this collaborative effort is to maintain scientific accuracy in the reporting of herbicide resistant weeds globally. The collaborative effort is supported by government, academic, and industry weed scientists worldwide. Moreover, the project is funded by the Global Herbicide Resistance Action Committee and CropLife International. The development of glyphosate resistance is a severe problem in agriculture because increasing amounts of the herbicide have to be applied in order to kill weed. Unfortunately, the increased usage of glyphosate may negatively affect the biodiversity because the weedkiller does not discriminate between weed and other plants that serve as a food source for insects like honey bees. Since honey bees are very important for pollination, a decrease of the honey bee population has a strong negative impact on plant reproduction, which also affects the fruit yield. Moreover, as crops as well as weed co-evolve with glyphosate over time, the development of herbicide resistance is never ending.
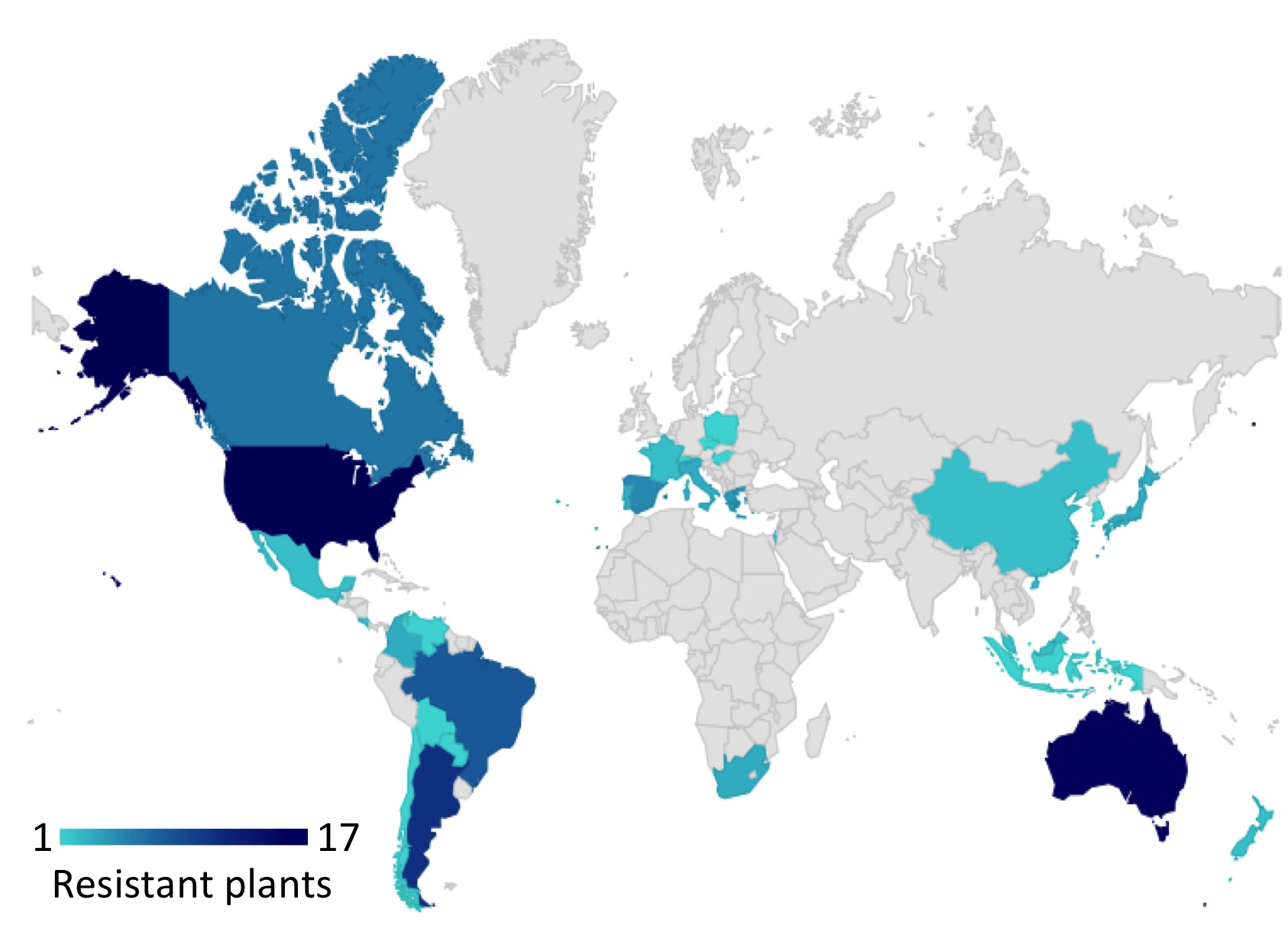
Figure 3. The development of resistance against glyphosate is increasing. Adapted from http://www.weedscience.org.
Other applications for glyphosate
Companies like Monsanto with patented chemical technologies will generally try to patent all reasonable potential uses of that chemical in order to obtain maximal return on their research investments. Since glyphosate inhibits the aromatic biosynthetic pathway in many bacteria and parasites, and a reasonable case can be made that glyphosate might be effective as an antimicrobial. Indeed, Monsanto has filed in 2003 for the invention that the herbicidal agent glyphosate can be used in combination with the polyvalent anion oxalic acid to prevent and treat pathogenic infections caused by protozoan parasites of the phylum Apicomplexa (Patent No.: US7,771,736B2). However, a lot stands between glyphosate as a substance with antimicrobial activity in the test tube and a clinically effective antimicrobial agent. A reliable effective concentration of glyphosate with a reasonable oral dose in humans is difficult to achieve, especially when glyphosate is orally applied. Moreover, glyphosate only shortly persists in humans. Glyphosate blocks the production of de novo synthesis of aromatic amino acids in bacteria, and the bacteria will die, or at least stop reproducing, if they cannot obtain these nutrients from the environment … but blood and tissues are not water - they are chock-full of the nutrients that microbes need to survive. The addition of aromatic amino acids to the growth medium of bacteria indeed interferes with glyphosate-dependent inhibition of the EPSP synthase (6). Moreover, nobody has demonstrated so far that glyphosate is an effective antimicrobial agent for treating human or animal infections. Therefore, it remains to be determined whether glyphosate may indeed act as an efficient and reliable antimicrobial agent to fight pathogenic bacteria and protozoan parasites.
The glyphosate controversy
Glyphosate is
The pros & cons of glyphosate usage in agriculture
Glyphosate is
References
- Amrhein et al. (1983) FEBS Lett. 157: 191-196.
- Comai et al. (1983) Science 221: 370-371.
- Schulz et al. (1984) Arch. Microbiol. 137: 121-123
- Steinrücken & Amrhein (1980) Biochem. Biophys. Res. Commun. 94: 1207-1212.
- Herrmann & Weaver (1999) Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 50: 473-503.
- Fischer et al. (1986) J. Bacteriol. 168: 1147-1154
- Gresshoff (1979) Aust. J. Plant. Physiol. 6: 177-185.
- Majumder et al. (1995) Eur. J. Biochem. 229: 99-106.
- Kononova & Nesmeyanova (2002) Biochemistry (Mosc) 67: 184-195.
- Li & Long (1988) Fundam. Appl. Toxicol. 10: 537-546.
- Duke & Powles (2008) Pest. Manag. Sci. 64: 319-325.
- Arjó et al. (2013) Transgenic Res. 22: 255-267.
- Benbrook (2016) Environ. Sci. Eur. 28: 3.
- Mesnage & Antoniou (2017) Front. Public. Health. 5: 316.
- Tincher et al. (2017) G3 (Bethesda). 7: 3331-3335.







