Bec Schacht (Talk | contribs) |
|||
| (20 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{Template:UNSW_Australia/Navbar}} | {{Template:UNSW_Australia/Navbar}} | ||
{{Template:UNSW_Australia/Header}} | {{Template:UNSW_Australia/Header}} | ||
| − | {{UNSW_Australia/Basics}} | + | {{Template:UNSW_Australia/Basics}} |
| + | {{Template:UNSW_Australia/Up}} | ||
<html> | <html> | ||
| Line 40: | Line 41: | ||
<h2>Introduction</h2> | <h2>Introduction</h2> | ||
| − | <p>Auxins are plant hormones (phytohormones) which are involved in the regulation of plant growth and development<sup>< | + | <p>Auxins are plant hormones (phytohormones) which are involved in the regulation of plant growth and development<sup><href="#references">1</sup>. Indole acetic acid (IAA) is the most widely characterised auxin, and the primary auxin found in most plant species<sup><href="#references">2</sup>. It is associated with many physiological processes in plants including cell elongation, cell division, tropisms to light and gravity, and root initiation and development<sup><href="#references">1</sup>.</p> |
| − | <p>Plants can naturally synthesise auxins, and the biosynthesis of IAA in <i>A. thaliana</i> occurs predominantly through a tryptophan dependent pathway<sup>< | + | <p>Plants can naturally synthesise auxins, and the biosynthesis of IAA in <i>A. thaliana</i> occurs predominantly through a tryptophan dependent pathway<sup><href="#references">3</sup>. Bacteria can also synthesise IAA through a variety of pathways, and often utilise IAA to interact with and colonise plants<sup><href="#references">4</sup>. To test our scaffold system with a proof of concept, we chose the two-step indole-3-acetamide pathway for IAA biosynthesis, the best characterised IAA biosynthetic pathway in bacteria<sup><href="#references">4</sup>.</p> |
| − | <p>Although plants can synthesise IAA <i>de novo</i>, application of exogenous phytohormones is common in industry to stimulate specific responses<sup>< | + | <p>Although plants can synthesise IAA <i>de novo</i>, application of exogenous phytohormones is common in industry to stimulate specific responses<sup><href="#references">5</sup>. Thus auxins, and phytohormones more broadly, have commercial significance. In order to understand the commercial significance of IAA we |
<a href=https://2018.igem.org/Team:UNSW_Australia/Human_Practices/Commercialisation target=_blank>consulted with real world users</a> of auxins and conducted experiments with the model plant <i>A. thaliana</i> to observe the effect of IAA on plant development. </p> | <a href=https://2018.igem.org/Team:UNSW_Australia/Human_Practices/Commercialisation target=_blank>consulted with real world users</a> of auxins and conducted experiments with the model plant <i>A. thaliana</i> to observe the effect of IAA on plant development. </p> | ||
| Line 55: | Line 56: | ||
<p>Wild type <i>A. thaliana</i> seeds were surface sterilised in a solution of 2.5% bleach and Tween for 10 minutes, and subsequently washed with sterile water. Seeds were then placed in a sterile solution of water and 1% sucrose and left to stratify at 4°C for two days. Seed germination occurred in the 1% sucrose solution at room temperature. </p> | <p>Wild type <i>A. thaliana</i> seeds were surface sterilised in a solution of 2.5% bleach and Tween for 10 minutes, and subsequently washed with sterile water. Seeds were then placed in a sterile solution of water and 1% sucrose and left to stratify at 4°C for two days. Seed germination occurred in the 1% sucrose solution at room temperature. </p> | ||
| − | <p>Germinated four-day old seedlings were transferred to square MS agar plates supplemented with 1% sucrose, containing IAA concentrations of 0 µM, 1 µM, 10 µM and 100 µM. Plates at each concentration of IAA were prepared in triplicate with approximately five seedlings placed in a line 2 cm from the edge of the plate. The plates were partially covered in foil, leaving only the top 2 cm exposed in order to limit IAA photodegradation and simulate the natural growth conditions of higher plants whereby only shoots are exposed to light<sup>< | + | <p>Germinated four-day old seedlings were transferred to square MS agar plates supplemented with 1% sucrose, containing IAA concentrations of 0 µM, 1 µM, 10 µM and 100 µM. Plates at each concentration of IAA were prepared in triplicate with approximately five seedlings placed in a line 2 cm from the edge of the plate. The plates were partially covered in foil, leaving only the top 2 cm exposed in order to limit IAA photodegradation and simulate the natural growth conditions of higher plants whereby only shoots are exposed to light<sup><href="#references">7</sup>. Plates were stored upright, illuminated and incubated at room temperature for 20 days. Primary root length and lateral root numbers were measured at four, six, eleven and twenty days following the initial seedling transplantation <b>(Figure 1)</b>. Detailed protocols can be found <a target="_blank" href="https://2018.igem.org/Team:UNSW_Australia/Experiments">on our experiments page</a>.</p> |
<div class=image-box> | <div class=image-box> | ||
| Line 64: | Line 65: | ||
<h2>Results</h2> | <h2>Results</h2> | ||
| − | <p>Primary root growth was inhibited in IAA concentrations from 1 to 100 µM, with the control 0 µM specimens displaying the longest mean primary root length (Figure 2).</p> | + | <p>Primary root growth was inhibited in IAA concentrations from 1 to 100 µM, with the control 0 µM specimens displaying the longest mean primary root length <b>(Figure 2)</b>.</p> |
<div class=image-box> | <div class=image-box> | ||
<img src=https://static.igem.org/mediawiki/2018/9/94/T--UNSW_Australia--plants-primary.png> | <img src=https://static.igem.org/mediawiki/2018/9/94/T--UNSW_Australia--plants-primary.png> | ||
| Line 70: | Line 71: | ||
<p class=figure-legend><b>Figure 2:</b> Effect of IAA concentration on <i>A. thaliana</i> seedling primary root length.<br /><sup>a</sup> Time elapsed since seedling transplanted to MS agar plate containing IAA.</p> | <p class=figure-legend><b>Figure 2:</b> Effect of IAA concentration on <i>A. thaliana</i> seedling primary root length.<br /><sup>a</sup> Time elapsed since seedling transplanted to MS agar plate containing IAA.</p> | ||
| − | <p> Conversely, lateral root count was highest in specimens grown in the media containing the highest, 100 µM concentration of IAA (Figure 3).</p> | + | <p> Conversely, lateral root count was highest in specimens grown in the media containing the highest, 100 µM concentration of IAA <b>(Figure 3)</b>.</p> |
<div class=image-box> | <div class=image-box> | ||
| Line 77: | Line 78: | ||
<p class="figure-legend"><b>Figure 3:</b> Effect of IAA concentration on <i>A. thaliana</i> lateral root count.<br /><sup>a</sup> Time elapsed since seedling transplanted to MS agar plate containing IAA.</p> | <p class="figure-legend"><b>Figure 3:</b> Effect of IAA concentration on <i>A. thaliana</i> lateral root count.<br /><sup>a</sup> Time elapsed since seedling transplanted to MS agar plate containing IAA.</p> | ||
| − | <p> Generally, plant growth (including shoots and leaf growth) was inhibited as the concentration of IAA was increased (Figure 4) | + | <p> Generally, plant growth (including shoots and leaf growth) was inhibited as the concentration of IAA was increased <b>(Figure 4)</b>.</p> |
<div class=flex-center> | <div class=flex-center> | ||
<div class=image-box> | <div class=image-box> | ||
| − | <img | + | <img src=https://static.igem.org/mediawiki/2018/5/55/T--UNSW_Australia--plants--compare2.png> |
</div> | </div> | ||
<div class=image-box> | <div class=image-box> | ||
| − | <img src=https://static.igem.org/mediawiki/2018/ | + | <img src=https://static.igem.org/mediawiki/2018/e/e4/T--UNSW_Australia--plants--compare1.png> |
</div> | </div> | ||
</div> | </div> | ||
| Line 92: | Line 93: | ||
<h3> Effect of IAA on <i>Arabidopsis thaliana</i> growth </h3> | <h3> Effect of IAA on <i>Arabidopsis thaliana</i> growth </h3> | ||
| − | <p>The effect of auxin on plant growth and development varies with auxin concentration<sup>< | + | <p>The effect of auxin on plant growth and development varies with auxin concentration<sup><href="#references">4</sup><sup>,</sup><sup><href="#references">5</sup>. |
| − | At high concentration auxins can have an inhibitory effect on cell elongation<sup>< | + | At high concentration auxins can have an inhibitory effect on cell elongation<sup><href="#references">1</sup>. The inhibitory effect of IAA on cell elongation at higher concentrations is thought to be due to the IAA-induced ethylene production, as ethylene has previously been shown to inhibit root elongation in <i>A. thaliana</i> <sup><href="#references">8</sup>. At lower concentrations, IAA has been shown to promote root elongation<sup><a href="#references">1</a></sup>, however this effect was not observed in our results <b>(Figure 2)</b>.</p> |
| − | <p>The promotion of lateral root formation at high concentrations of IAA is consistent with previous literature which has shown that IAA promotes, and is essential for lateral root development in <i>A. thaliana</i> <sup>< | + | <p>The promotion of lateral root formation at high concentrations of IAA is consistent with previous literature which has shown that IAA promotes, and is essential for lateral root development in <i>A. thaliana</i> <sup><href="#references">9</sup><sup>,</sup><sup><href="#references">10</sup>.</p> |
| − | <p>Overall, the control specimens that were grown with no IAA appeared to exhibit the best development, with consistently greater leaf and shoot growth (Figure 3). In contrast, the decreased root elongation and increased formation of lateral roots observed in the specimens frown with the addition of 100 µM IAA is similar to a stress-induced morphogenic response of <i>A. thaliana</i> <sup>< | + | <p>Overall, the control specimens that were grown with no IAA appeared to exhibit the best development, with consistently greater leaf and shoot growth <b>(Figure 3)</b>. In contrast, the decreased root elongation and increased formation of lateral roots observed in the specimens frown with the addition of 100 µM IAA is similar to a stress-induced morphogenic response of <i>A. thaliana</i> <sup><href="#references">11</sup>.</p> |
<h3>IAA (in)stability </h3> | <h3>IAA (in)stability </h3> | ||
| − | <p>Our results indicate that exogenous IAA facilitated lateral root sprouting but inhibited primary root elongation at the concentrations that we used. This is consistent with the literature and with comments made by researchers at the PlantBank facilities whom we consulted with. Auxins are used by researchers at PlantBank, typically to stimulate adventitious root growth in their tissue culture specimens. Adventitious root growth in this context refers to roots which have developed from an unusual location (i.e. leaves or shoots)<sup>< | + | <p>Our results indicate that exogenous IAA facilitated lateral root sprouting but inhibited primary root elongation at the concentrations that we used. This is consistent with the literature and with comments made by researchers at the PlantBank facilities whom we consulted with. Auxins are used by researchers at PlantBank, typically to stimulate adventitious root growth in their tissue culture specimens. Adventitious root growth in this context refers to roots which have developed from an unusual location (i.e. leaves or shoots)<sup><href="#references">12</sup> and allows for cultured specimens grown in the laboratory to be transplanted into soil and grown outside. This has commercial significance as tissue cultured specimens must be rooted and established in soil to be sold to a retail consumer.</p> |
<p>However, researchers at the PlantBank facility exclusively use indole-3-butyric acid (IBA), another auxin, as they had found in previous experiments that IAA showed no benefits and that IBA was easier to work with.</p> | <p>However, researchers at the PlantBank facility exclusively use indole-3-butyric acid (IBA), another auxin, as they had found in previous experiments that IAA showed no benefits and that IBA was easier to work with.</p> | ||
| − | <p>These findings may be partially explained by the relative instability of IAA when compared to IBA. Indole-3-aecetic acid is sensitive to photodegradation, which can be accelerated by the minerals present in MS media<sup>< | + | <p>These findings may be partially explained by the relative instability of IAA when compared to IBA. Indole-3-aecetic acid is sensitive to photodegradation, which can be accelerated by the minerals present in MS media<sup><href="#references">13</sup>. Although IBA is also subject to photodegradation, the effects are less than that of IAA with tests conducted in MS media finding concentrations of IAA were reduced by more than 97% after 20 days in the light compared to a 60% reduction in IBA<sup><href="#references">14</sup>.</p> |
<p>To account for light sensitivity of IAA, our experimental design was adapted to minimise IAA photodegradation. We conducted all media preparation and plating in a dark laminar flow hood and partially covered our plant samples during growth.</p> | <p>To account for light sensitivity of IAA, our experimental design was adapted to minimise IAA photodegradation. We conducted all media preparation and plating in a dark laminar flow hood and partially covered our plant samples during growth.</p> | ||
| Line 124: | Line 125: | ||
<h3>Future Directions </h3> | <h3>Future Directions </h3> | ||
| − | <p>Due to time constraints, we were not able to conduct experiments with IAA synthesised using our scaffold. However, testing the effects of IAA purchased from Sigma Aldrich allowed us to begin developing a <a target="_blank" href="https://2018.igem.org/Team:UNSW_Australia/Experiments">protocol</a> which can be further refined into the future. Primary root growth decreased with increasing IAA concentration which may indicate that the concentrations of IAA used were too high, as reports in the literature state that too much exogenous IAA can inhibit root elongation<sup>< | + | <p>Due to time constraints, we were not able to conduct experiments with IAA synthesised using our scaffold. However, testing the effects of IAA purchased from Sigma Aldrich allowed us to begin developing a <a target="_blank" href="https://2018.igem.org/Team:UNSW_Australia/Experiments">protocol</a> which can be further refined into the future. Primary root growth decreased with increasing IAA concentration which may indicate that the concentrations of IAA used were too high, as reports in the literature state that too much exogenous IAA can inhibit root elongation<sup><href="#references">1</sup>. Therefore, if we are to re-attempt this experiment in the future we will first have to determine the optimal concentration of IAA to use for our plants</p> |
<div id="references"> | <div id="references"> | ||
| Line 130: | Line 131: | ||
<ol> | <ol> | ||
| − | <li> Davies, P. J. Plant Hormones: Biosynthesis, Signal Transduction, Action! | + | <li> Davies, P. J. Plant Hormones: Biosynthesis, Signal Transduction, Action! 1-800 (Springer Netherlands, 2007).</li> |
| − | <li> Gray, W. M. Hormonal regulation of plant growth and development. <i>PLoS Biol</i> <b>2</b> | + | <li> Gray, W. M. Hormonal regulation of plant growth and development. <i>PLoS Biol</i>. <b>2</b> e311, (2004).</li> |
| − | <li> Mashiguchi, K. et al. The main auxin biosynthesis pathway in <i>Arabidopsis. Proc Natl Acad Sci U S A</i> <b>108</b> | + | <li> Mashiguchi, K. et al. The main auxin biosynthesis pathway in <i>Arabidopsis. Proc Natl Acad Sci U S A</i>. <b>108</b> 18512-18517 (2011).</li> |
| − | <li> Spaepen, S., Vanderleyden, J. | + | <li> Spaepen, S., Vanderleyden, J. and Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. <i>FEMS Microbiol Rev</i>. <b>31</b> 425-448 (2007).</li> |
<li> Szajdak, L. W. Bioactive Compounds in Agricultural Soils. (Springer International Publishing, 2016).</li> | <li> Szajdak, L. W. Bioactive Compounds in Agricultural Soils. (Springer International Publishing, 2016).</li> | ||
| − | <li> Loyola-Vargas, V. M. | + | <li> Loyola-Vargas, V. M. and Vazquez-Flota, F. An introduction to plant cell culture: Back to the future. <i>Methods Mol Biol</i>. <b>318</b> 3-8 (2006).</li> |
| − | <li> Xu, W. et al. An improved agar-plate method for studying root growth and response of Arabidopsis thaliana. <i>Sci Rep</i> <b>3</b> | + | <li> Xu, W. et al. An improved agar-plate method for studying root growth and response of Arabidopsis thaliana. <i>Sci Rep</i>. <b>3</b> 1273 (2013).</li> |
| − | <li> Ruzicka, K. et al. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution.<i>Plant Cell</i> <b>19</b> | + | <li> Ruzicka, K. et al. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. <i>Plant Cell</i>. <b>19</b> 2197-2212 (2007).</li> |
| − | <li> Celenza, J. L., Jr., Grisafi, P. L. | + | <li> Celenza, J. L., Jr., Grisafi, P. L. and Fink, G. R. A pathway for lateral root formation in <i>Arabidopsis thaliana. Genes Dev</i>. <b>9</b> 2131-2142 (1995).</li> |
| − | <li> Reed, R. C., Brady, S. R. | + | <li> Reed, R. C., Brady, S. R. and Muday, G. K. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. <i>Plant Physiol</i> <b>118</b> 1369-1378 (1998).</li> |
| − | <li> Potters, G. | + | <li> Potters, G. et al. Stress-induced morphogenic responses: growing out of trouble? <i>Trends Plant Sci</i>. <b>12</b> 98-105 (2007).</li> |
| − | <li> Davis, T. D. | + | <li> Davis, T. D. and Haissig, B. E. Biology of Adventitious Root Formation. (Springer US, 2013).</li> |
| − | <li> Dunlap, J. R. | + | <li> Dunlap, J. R. and Robacker, K. M. Nutrient salts promote light-induced degradation of indole-3-acetic Acid in tissue culture media. <i>Plant Physiol</i>. <b>88</b> 379-382 (1988).</li> |
| − | <li> Nissen, S. J. | + | <li> Nissen, S. J. and Sutton, E.J. Stability of IAA and IBA in nutrient medium to several tissue culture procedures. <i>HortScience</i>. <b>25</b> 800-802 (1990).</li> |
</ol> | </ol> | ||
</div> | </div> | ||
</html> | </html> | ||
Latest revision as of 02:41, 18 October 2018


Plants
Overview
Auxins are plant hormones which are involved in the regulation of plant growth and development. The biosynthesis of the auxin indole-3-aecetic acid (IAA) was used as a test pathway for our scaffold system, thus a protocol was developed to investigate the functionality of biosynthetically produced IAA in a plant growth assay compared to commercially available IAA. To this aim, Arabidopsis thaliana seedlings were grown in media containing varying concentrations of IAA to observe its effect on plant growth and development. Increased lateral root growth was observed in seedlings exposed to increased concentrations of exogenous IAA, which is consistent to other reports in the literature. At the same time, primary root growth was decreased alongside increased IAA concentration. This may indicate that the concentrations of IAA used were too high, as again this matches reports in the literature that too much exogenous IAA can inhibit root elongation. Therefore, if we are to re-attempt this experiment in the future we will first have to determine the optimal concentration of IAA to use for our plants. Nevertheless, we have developed a protocol that could be used in the future to observe the functionality of the IAA product produced with our scaffold compared with commercially available IAA.
Introduction
Auxins are plant hormones (phytohormones) which are involved in the regulation of plant growth and development
Plants can naturally synthesise auxins, and the biosynthesis of IAA in A. thaliana occurs predominantly through a tryptophan dependent pathway
Although plants can synthesise IAA de novo, application of exogenous phytohormones is common in industry to stimulate specific responses
Aims
To observe the effect of the auxin indole-3-acetic acid on Arabidopsis thaliana growth and development.
Methods
Wild type A. thaliana seeds were surface sterilised in a solution of 2.5% bleach and Tween for 10 minutes, and subsequently washed with sterile water. Seeds were then placed in a sterile solution of water and 1% sucrose and left to stratify at 4°C for two days. Seed germination occurred in the 1% sucrose solution at room temperature.
Germinated four-day old seedlings were transferred to square MS agar plates supplemented with 1% sucrose, containing IAA concentrations of 0 µM, 1 µM, 10 µM and 100 µM. Plates at each concentration of IAA were prepared in triplicate with approximately five seedlings placed in a line 2 cm from the edge of the plate. The plates were partially covered in foil, leaving only the top 2 cm exposed in order to limit IAA photodegradation and simulate the natural growth conditions of higher plants whereby only shoots are exposed to light

Figure 1: Summary of methods used for plant growth assay
Results
Primary root growth was inhibited in IAA concentrations from 1 to 100 µM, with the control 0 µM specimens displaying the longest mean primary root length (Figure 2).

Figure 2: Effect of IAA concentration on A. thaliana seedling primary root length.
a Time elapsed since seedling transplanted to MS agar plate containing IAA.
Conversely, lateral root count was highest in specimens grown in the media containing the highest, 100 µM concentration of IAA (Figure 3).
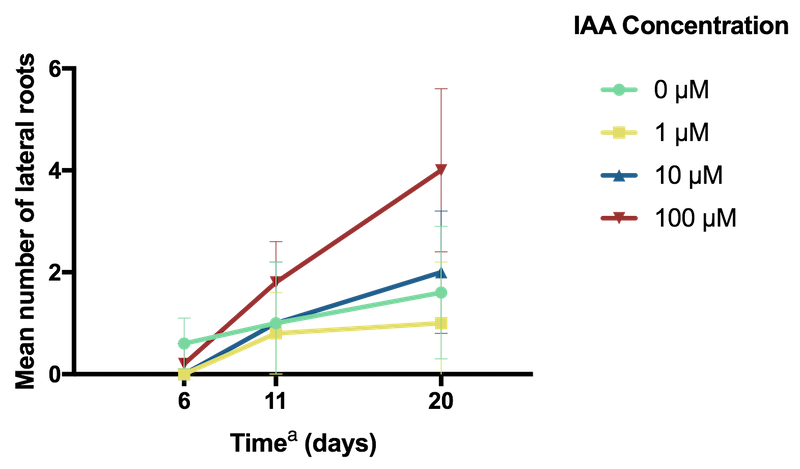
Figure 3: Effect of IAA concentration on A. thaliana lateral root count.
a Time elapsed since seedling transplanted to MS agar plate containing IAA.
Generally, plant growth (including shoots and leaf growth) was inhibited as the concentration of IAA was increased (Figure 4).


Figure 4: Arabidopsis thaliana seedlings growth after 20 days incubation, illuminated at room temperature on MS agar with no IAA (left) and 100µM IAA concentration (right).
Discussion
Effect of IAA on Arabidopsis thaliana growth
The effect of auxin on plant growth and development varies with auxin concentration
The promotion of lateral root formation at high concentrations of IAA is consistent with previous literature which has shown that IAA promotes, and is essential for lateral root development in A. thaliana
Overall, the control specimens that were grown with no IAA appeared to exhibit the best development, with consistently greater leaf and shoot growth (Figure 3). In contrast, the decreased root elongation and increased formation of lateral roots observed in the specimens frown with the addition of 100 µM IAA is similar to a stress-induced morphogenic response of A. thaliana
IAA (in)stability
Our results indicate that exogenous IAA facilitated lateral root sprouting but inhibited primary root elongation at the concentrations that we used. This is consistent with the literature and with comments made by researchers at the PlantBank facilities whom we consulted with. Auxins are used by researchers at PlantBank, typically to stimulate adventitious root growth in their tissue culture specimens. Adventitious root growth in this context refers to roots which have developed from an unusual location (i.e. leaves or shoots)
However, researchers at the PlantBank facility exclusively use indole-3-butyric acid (IBA), another auxin, as they had found in previous experiments that IAA showed no benefits and that IBA was easier to work with.
These findings may be partially explained by the relative instability of IAA when compared to IBA. Indole-3-aecetic acid is sensitive to photodegradation, which can be accelerated by the minerals present in MS media
To account for light sensitivity of IAA, our experimental design was adapted to minimise IAA photodegradation. We conducted all media preparation and plating in a dark laminar flow hood and partially covered our plant samples during growth.


Figure 5: Experimental set up of plants to minimise IAA photodegradation.
Implications for Commercialisation with our scaffold
After learning of the exclusive use of IBA rather than IAA for exogenous application of auxin in tissue culture at PlantBank, in combination with the results of our experimental attempts to observe the effects of IAA on A. thaliana growth and development, we determined that using this method to assess the effect of exogenous IAA on plant root growth requires further optimisation. In particular, the concentration of IAA needed to provide a beneficial rather than inhibitory effect on root growth needs to be determined. Nevertheless, we observed IAA having an effect on our plant roots, and with future optimisation we will be able to compare the effect of commercially available IAA to the effect of IAA biosynthesised with our Assemblase scaffold.
Future Directions
Due to time constraints, we were not able to conduct experiments with IAA synthesised using our scaffold. However, testing the effects of IAA purchased from Sigma Aldrich allowed us to begin developing a protocol which can be further refined into the future. Primary root growth decreased with increasing IAA concentration which may indicate that the concentrations of IAA used were too high, as reports in the literature state that too much exogenous IAA can inhibit root elongation
References
- Davies, P. J. Plant Hormones: Biosynthesis, Signal Transduction, Action! 1-800 (Springer Netherlands, 2007).
- Gray, W. M. Hormonal regulation of plant growth and development. PLoS Biol. 2 e311, (2004).
- Mashiguchi, K. et al. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A. 108 18512-18517 (2011).
- Spaepen, S., Vanderleyden, J. and Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 31 425-448 (2007).
- Szajdak, L. W. Bioactive Compounds in Agricultural Soils. (Springer International Publishing, 2016).
- Loyola-Vargas, V. M. and Vazquez-Flota, F. An introduction to plant cell culture: Back to the future. Methods Mol Biol. 318 3-8 (2006).
- Xu, W. et al. An improved agar-plate method for studying root growth and response of Arabidopsis thaliana. Sci Rep. 3 1273 (2013).
- Ruzicka, K. et al. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 19 2197-2212 (2007).
- Celenza, J. L., Jr., Grisafi, P. L. and Fink, G. R. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 9 2131-2142 (1995).
- Reed, R. C., Brady, S. R. and Muday, G. K. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118 1369-1378 (1998).
- Potters, G. et al. Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 12 98-105 (2007).
- Davis, T. D. and Haissig, B. E. Biology of Adventitious Root Formation. (Springer US, 2013).
- Dunlap, J. R. and Robacker, K. M. Nutrient salts promote light-induced degradation of indole-3-acetic Acid in tissue culture media. Plant Physiol. 88 379-382 (1988).
- Nissen, S. J. and Sutton, E.J. Stability of IAA and IBA in nutrient medium to several tissue culture procedures. HortScience. 25 800-802 (1990).

