| Line 127: | Line 127: | ||
<div class="center"> | <div class="center"> | ||
<figure id="figure7" class="figure" style="text-align:center;"> | <figure id="figure7" class="figure" style="text-align:center;"> | ||
| − | <img style="width : | + | <img style="width : 45%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/8/85/T--Toulouse-INSA-UPS--Results--Youn--bactcell.png" class="figure-img img-fluid rounded" alt="Fluorescence Retained"> |
<figcaption id="figcaption7" class="figure-caption"><strong>Figure 2:</strong> <i>Picture of functionalized cellulose with Sirius (CBM3a fused with mRFP1). Left: control mRFP1 alone; Right: Sirius </i></figcaption> | <figcaption id="figcaption7" class="figure-caption"><strong>Figure 2:</strong> <i>Picture of functionalized cellulose with Sirius (CBM3a fused with mRFP1). Left: control mRFP1 alone; Right: Sirius </i></figcaption> | ||
</figure> | </figure> | ||
Revision as of 15:27, 16 October 2018
DEMONSTRATE
The integration of our entrepreneurial and social analysis led us to consider finding a way to functionalize bacterial cellulose. For this purpose, we designed a three headed proteic platform that we named Cerberus. It is composed of a cellulose-binding molecular module (CBM3a) fused at its N-terminal part to a biotinylated molecule-binding head (monomeric streptavidin) and at its C-terminal part to an unnatural amino acid (UnAA) 4-azido-L-phenylalanine moiety. We also successfully set up a workflow allowing the synthesis of Cerberus (Figure 1). Our procedure was divided in two steps: validation of the system and cellulose functionalization.


Validation of the Three Binding Heads
 Cellulose Binding
Cellulose Binding
We designed a fusion protein between the Carbohydrate Binding Module type 3a and a fluorescent reporter and named it Sirius. Its purpose is to validate the association of our system towards cellulose. We demonstrated, using mRFP1 alone as negative control, the binding of our protein as showned in Figure 2. We were then confident in the binding of our protein to cellulose.

 Biotinylated compound affinity
Biotinylated compound affinity
Our second construction consisted on our Cerberus protein but lacking the unnatural amino acid. This construction aimed to prove the binding affinity of our system toward biotinylated compounds. As biotinylation can be both performed at high yield in vivo and in vitro, this construction is already a molecular binding plateform, allowing the purification of biotinylated compounds at low cost, using cellulose. We validated this construction using biotinylated mTag Blue Fluorescent Protein (Figure 3.a), biotinylated in vivo in E. coli.

 Click chemistry
Click chemistry
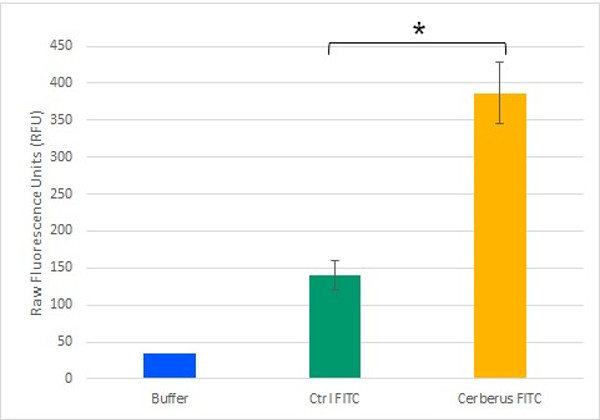
Our plateform aims to have a binding capacity as wide as possible. The azide groupment offers a high efficiency covalent bound formation through cycloaddition reaction, mostyl known by Click chemistry. The possibilities offered by this system are therefore included in the unnatural amino acid : 4-azido-L-phenylalanine. We designed our protein to bear an Amber stop codon for unnatural amino acid integration in our plateform. We confirmed the activity of this integration using DBCO-Fluorescein as showed in figure 4.
Compounds Fuctionalization
 Fluorescence
Fluorescence
Once our system proved, we tried to associate several molecules in order to prove the system versatility. We successfully associate fluorescein to the streptavidin head. We made it possible thanks to the click chemistry with which we brought the necessary biotin for the streptavidin interaction.


 Color
Color
Thanks to Sirius and its brightness, we could observe how the color remained on Bacterial Cellulose (figure 2) even after dozen of washes!


 Antibacterial Activity
Antibacterial Activity
We also conjugated an antimicrobial peptide, scygonadin, to the streptavidin, even if we need to optimize protocol (figure 3) we are comfident in the remaining biological activity after peptide binding on Cerberus.

 Paramagnetism
Paramagnetism
For the AzF head, we decided to bind an inorganic molecule: paramagnetic beads, and it was a success (see the video below, figure 4). So our Cerberus platform has allowed us to make magnetic cellulose.

 Versatility of the system
Versatility of the system
All of our experiments demonstrated the versatility of our system. Indeed, click chemistry is a real advantage to increase number of possibilities of functionalities. DBCO-biotin compound may be used as an adaptor between the streptavidin head and the AzF head as showed in figure 6.


Perspectives and conclusion
We successfully set up a workflow allowing the synthesis of Cerberus, a proteic platform that is composed of a cellulose-binding molecular module (CBM3a) fused at its N-terminal part to a biotinylated molecule-binding head (monomeric streptavidin) and at its C-terminal part to an unnatural amino acid (UnAA) 4-azido-L-phenylalanine moiety. Combining molecular modeling, synthetic biology and chemistry, we proved that Cerberus is a valuable and versatile platform allowing to functionalize cellulose with organic and inorganic compounds.
We can also plan to associate carbon nanotubes and graphene to AzF head in the aim to change cellulose fibre rigidity and create conductive cellulose, respectively. In order to do that, we successfully activated these compounds by diazotization reaction. The click chemistry assay and validation protocol need improvement to obtain our goal.
Bacterial cellulose has unique properties such as its natural purity may give it a bright future in the industries, that is why we decided to produce functionalized bacterial cellulose in vivo in reactor. As a first step towards the production of functionalized bacterial cellulose in a reactor, we demonstrate that CBM3a module of Cerberus fused to Red fluorescent protein is able to make bacterial cellulose fluorescent. Moreover, our first production of Orthos in Pichia pastoris are very conclusive, but we have to optimize the production.
We truly feel that Cerberus platform is a very convenient and efficient solution to facilitate molecular work for iGEM projects and laboratory work in general. In fact, we can combine the use of two binding sites to purify simply molecules of interest (cellulose/streptavidin or cellulose/AzF or streptavidin/AzF). For example, we can imagine purify cells via biotinylated membrane proteins. An addition of Cerberus protein can permit the interaction between the monomeric streptavidin of Cerberus and the biotin of protein. Then, two possibilities are open to us to purify using either cellulose or paramagnetic beads. After attraction, we can release molecule of interest by using the TEV protease cleavage.
Finally, we can also imagine to change our CBM3a to another proteic domain to associate functionalizing proteins to new materials as plastic to facilitate its degradation for example.
No dogs were harmed over the course of this iGEM project.
The whole Toulouse INSA-UPS team wants to thank our sponsors, especially:









And many more. For futher information about our sponsors, please consult our Sponsors page.
The content provided on this website is the fruit of the work of the Toulouse INSA-UPS iGEM Team. As a deliverable for the iGEM Competition, it falls under the Creative Commons Attribution 4.0. Thus, all content on this wiki is available under the Creative Commons Attribution 4.0 license (or any later version). For futher information, please consult the official website of Creative Commons.
This website was designed with Bootstrap (4.1.3). Bootstrap is a front-end library of component for html, css and javascript. It relies on both Popper and jQuery. For further information, please consult the official website of Bootstrap.



