Kasparas12 (Talk | contribs) |
Kristinazu (Talk | contribs) |
||
| Line 201: | Line 201: | ||
</p> | </p> | ||
<p> | <p> | ||
| − | + | <div class="image-container"> | |
| − | </ | + | <img src="https://static.igem.org/mediawiki/2018/d/d1/T--Vilnius-Lithuania--Fig1_Mistic.png"/> |
| + | <p><strong>Fig. 1 </strong> NMR structure of Mistic (MstX). Protein is comprised of four ɑ-helices with a polar lipid-facing surface. Topology measurements have shown that both C-terminus and N-terminus of MstX are exposed at the same side. Adapted by Yarnell, 2005</p> | ||
| + | </div> | ||
<p> | <p> | ||
MstX was identified back in 2005 by Rooslid and colleagues. Interestingly, until this day little is known about how MstX promotes integral protein targeting to the membrane<sup>3</sup>. Recently it has found a novel application as a fusion tag supporting the recombinant production and bilayer insertion of other membrane proteins (MPs)<sup>1</sup>. MstX, when fused to the N-terminus of integral MPs, enables the cargo proteins to fold into their native conformations in the membrane, thus yielding high-level expression. It is known that MstX autonomously targets proteins to the membrane bypassing the canonical secretory apparatus, like Sec translocon. In addition to this, it was indirectly presumed that MstX lacks any recognizable signal sequence <sup>2</sup>. | MstX was identified back in 2005 by Rooslid and colleagues. Interestingly, until this day little is known about how MstX promotes integral protein targeting to the membrane<sup>3</sup>. Recently it has found a novel application as a fusion tag supporting the recombinant production and bilayer insertion of other membrane proteins (MPs)<sup>1</sup>. MstX, when fused to the N-terminus of integral MPs, enables the cargo proteins to fold into their native conformations in the membrane, thus yielding high-level expression. It is known that MstX autonomously targets proteins to the membrane bypassing the canonical secretory apparatus, like Sec translocon. In addition to this, it was indirectly presumed that MstX lacks any recognizable signal sequence <sup>2</sup>. | ||
| Line 213: | Line 215: | ||
</p> | </p> | ||
<p> | <p> | ||
| − | + | <div class="image-container"> | |
| − | + | <img src="https://static.igem.org/mediawiki/2018/f/fa/T--Vilnius-Lithuania--Fig2_Mistic.png"/> | |
| − | + | <p><strong>Fig. 2 </strong> OmpA-X and MstX-OmpA-X (X - additional part of the construct; shown with arrows) expression in <var>E. coli</var>; SDS-PAGE after induction with IPTG for 2 hours and 4 hours; K - control, M - protein ladder </p> | |
| + | </div> | ||
| + | |||
Also, we checked if we could fuse MstX with other integral membrane proteins. In this case, IgA protein (Fig. 3). | Also, we checked if we could fuse MstX with other integral membrane proteins. In this case, IgA protein (Fig. 3). | ||
</p> | </p> | ||
| − | + | <p> | |
| − | + | <div class="image-container"> | |
| + | <img src="https://static.igem.org/mediawiki/2018/3/31/T--Vilnius-Lithuania--Fig3_Mistic.png"/> | ||
| + | <p><strong>Fig. 3 </strong> IgA, IgA-MstX, and X-IgA-MstX (X - additional part of the construct; shown with arrows) expression in <var>E. coli</var>; SDS-PAGE; U4-2 - samples affected with 8M urea, S2-4 - samples affected with protein denaturation dye, K - control, M - protein ladder </p> | ||
| + | </div> | ||
</p> | </p> | ||
<p> | <p> | ||
| Line 234: | Line 241: | ||
</p> | </p> | ||
<p> | <p> | ||
| − | + | <div class="image-container"> | |
| − | </ | + | <img src="https://static.igem.org/mediawiki/2018/7/79/T--Vilnius-Lithuania--Fig4_Mistic.png"/> |
| + | <p><strong>Fig. 4 </strong> Single-chain variable fragment (scFv) expression in IVTT system; SDS-PAGE. M - protein ladder, + - positive control DHFR, 1 - scFv, 2 - MstX-scFv, - negative control (without template DNA)</p> | ||
| + | </div> | ||
<p> | <p> | ||
Analyzing the reaction samples, sediments in scFv were observed, which meant that scFv aggregated. However, in MstX-scFv sample there were no sediments. By analysing electrophoresis results (Fig. 4) it can be seen that MstX prevented formation of the aggregates which resulted in higher scFv expression yield. | Analyzing the reaction samples, sediments in scFv were observed, which meant that scFv aggregated. However, in MstX-scFv sample there were no sediments. By analysing electrophoresis results (Fig. 4) it can be seen that MstX prevented formation of the aggregates which resulted in higher scFv expression yield. | ||
Revision as of 22:18, 17 October 2018
Design and Results
Results
Cell-free, synthetic biology systems open new horizons in engineering biomolecular systems which feature complex, cell-like behaviors in the absence of living entities. Having no superior genetic control, user-controllable mechanisms to regulate gene expression are necessary to successfully operate these systems. We have created a small collection of synthetic RNA thermometers that enable temperature-dependent translation of membrane proteins, work well in cells and display great potential to be transferred to any in vitro protein synthesis system.



 Fig. 2 Electrophoresis gel of PCR products: 6 - Sw2, 7 - Sw3, 8 - Sw6, 9 - Sw7, 10 - Sw9, 11 - Sw11.
Fig. 2 Electrophoresis gel of PCR products: 6 - Sw2, 7 - Sw3, 8 - Sw6, 9 - Sw7, 10 - Sw9, 11 - Sw11.
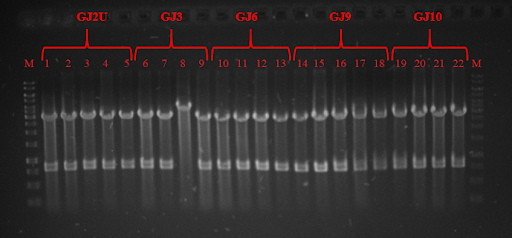 Fig. 3 Restriction analysis of GJx constructs
Fig. 3 Restriction analysis of GJx constructs
 Fig. 4 Colony PCR of RNA thermometers in pSB1C3 plasmid.
Fig. 4 Colony PCR of RNA thermometers in pSB1C3 plasmid.
 Fig. 5 expression at 24 ˚C. On the right you can see GFP expression without RNA thermometer.
Fig. 5 expression at 24 ˚C. On the right you can see GFP expression without RNA thermometer.
 Fig. 6 GFP expression at 30 ˚C. On the right you can see GFP expression without RNA thermometer.
Fig. 6 GFP expression at 30 ˚C. On the right you can see GFP expression without RNA thermometer.
 Fig. 7 GFP expression in 37 ˚C. On the right you can see GFP expression without RNA thermometer.
Fig. 7 GFP expression in 37 ˚C. On the right you can see GFP expression without RNA thermometer.
 Fig. 8 Associational scheme of thermoswitches’ action in the SynDrop system. Not locking the concomitant translation of our target protein and BamA results in target protein aggregation due to insufficient membrane insertion and assembling potential of BamA.
Fig. 8 Associational scheme of thermoswitches’ action in the SynDrop system. Not locking the concomitant translation of our target protein and BamA results in target protein aggregation due to insufficient membrane insertion and assembling potential of BamA.
 Fig. 9 Associational scheme of thermoswitches’ action in the SynDrop system. Locking up translation gives time for proper folding and insertion of BamA and prevents undesirable aggregation of target membrane proteins.
Fig. 9 Associational scheme of thermoswitches’ action in the SynDrop system. Locking up translation gives time for proper folding and insertion of BamA and prevents undesirable aggregation of target membrane proteins.




 Fig. 1 Simplified structure of scFv Antibody
Fig. 1 Simplified structure of scFv Antibody
 Fig. 2 Scheme of scFv_antiVLY and VLY interaction. Left- scFv_antiVLY binds to VLY, erythrocytes stay intact, Right- scFv_antiVLY does not bind and VLY lyse erythrocytes.
Fig. 2 Scheme of scFv_antiVLY and VLY interaction. Left- scFv_antiVLY binds to VLY, erythrocytes stay intact, Right- scFv_antiVLY does not bind and VLY lyse erythrocytes.
 Fig. 3 SDS-PAGE of scFv. GFP is used as positive control, C- chaperone DnaK.
Fig. 3 SDS-PAGE of scFv. GFP is used as positive control, C- chaperone DnaK.
 Fig. 4 Percentage of erythrocyte lysis at different +/-scFv dilutions.
Fig. 4 Percentage of erythrocyte lysis at different +/-scFv dilutions.
 Fig. 5 A- MstX-scFv_antiVLY expression in Escherichia coli. B- scFv_antiVLY and MstX-scFv_antiVLY expression in cell-free system.
Fig. 5 A- MstX-scFv_antiVLY expression in Escherichia coli. B- scFv_antiVLY and MstX-scFv_antiVLY expression in cell-free system.
 Fig. 6 Fig 6. Percentage of erythrocyte lysis at different scFv/MstX-scFv dilutions.
Fig. 6 Fig 6. Percentage of erythrocyte lysis at different scFv/MstX-scFv dilutions.