Kristinazu (Talk | contribs) |
Kristinazu (Talk | contribs) |
||
| Line 222: | Line 222: | ||
With custom IDT primers with overhangs bearing thermoswitch sequences we performed a PCR from a GFP gene containing plasmid and inserted RNA thermometers GJ<sub>x</sub> (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622010">BBa_K2622010</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622011">BBa_K2622011</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622012">BBa_K2622012</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622013">BBa_K2622013</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622014">BBa_K2622014</a>) upstream the GFP gene. Another set of primers was used to produce RNA thermometers Sw<sub>x</sub> (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622016">BBa_K2622016</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622017">BBa_K2622017</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622018">BBa_K2622018</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622019">BBa_K2622019</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622020">BBa_K2622020</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622021">BBa_K2622021</a>). PCR was successful and all products were the same size as expected for Sw<sub>x</sub> constructs ~76 bp (Fig. 2). DNA gel electrophoresis was not performed for GJx constructs, because whole plasmid was multiplied and only 40-60 bp were inserted. | With custom IDT primers with overhangs bearing thermoswitch sequences we performed a PCR from a GFP gene containing plasmid and inserted RNA thermometers GJ<sub>x</sub> (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622010">BBa_K2622010</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622011">BBa_K2622011</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622012">BBa_K2622012</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622013">BBa_K2622013</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622014">BBa_K2622014</a>) upstream the GFP gene. Another set of primers was used to produce RNA thermometers Sw<sub>x</sub> (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622016">BBa_K2622016</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622017">BBa_K2622017</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622018">BBa_K2622018</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622019">BBa_K2622019</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622020">BBa_K2622020</a>, <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622021">BBa_K2622021</a>). PCR was successful and all products were the same size as expected for Sw<sub>x</sub> constructs ~76 bp (Fig. 2). DNA gel electrophoresis was not performed for GJx constructs, because whole plasmid was multiplied and only 40-60 bp were inserted. | ||
</p> | </p> | ||
| − | <p | + | <p> |
<img src="https://static.igem.org/mediawiki/2018/c/ca/T--Vilnius-Lithuania--THERMO_fig_2.png"/> | <img src="https://static.igem.org/mediawiki/2018/c/ca/T--Vilnius-Lithuania--THERMO_fig_2.png"/> | ||
<strong>Fig. 2</strong> Electrophoresis gel of PCR products: 6 - Sw2, 7 - Sw3, 8 - Sw6, 9 - Sw7, 10 - Sw9, 11 - Sw11. | <strong>Fig. 2</strong> Electrophoresis gel of PCR products: 6 - Sw2, 7 - Sw3, 8 - Sw6, 9 - Sw7, 10 - Sw9, 11 - Sw11. | ||
</p> | </p> | ||
| − | |||
<p> | <p> | ||
pRSET plasmid and Sw<sub>x</sub> PCR products were digested with restriction enzymes and ligated, while GJ<sub>x</sub> PCR products were phosphorylated and ligated to produce plasmids from linear products. DH5α competent cells were transformed and plated on lysogeny broth (LB) media with ampicillin (Amp) and grown for 16 hours. Positive colonies were selected by colony PCR or restriction analysis (Fig. 3 and Fig. 4) and grown in 5 mL LB media. Plasmids were purified and BL21 competent cells were transformed. Three tubes of every construct plus plasmid with GFP without RNA thermometer were grown till OD<sub>600</sub> reached 0.4. Control samples were taken and protein expression was induced with Isopropyl β-D-1-thiogalactopyranoside (IPTG). One tube of every construct was grown in 24 ˚C, 30 ˚C, and 37 ˚C. Samples were taken after 1 and 2 hours. SDS-PAGE was run (for elaborate protocol see Notebook/<a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols">Protocols</a>). Fig. 5, Fig 6 and Fig. 7 depicts GFP expression at different temperatures. Although our RNA thermometers were designed to melt at 37 ˚C, some displayed leakiness to different extent. GJ3 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622011">BBa_K2622011</a>) RNA thermometer was the leakeast and allowed for GFP translation at lower temperatures. On the other hand, when grown at 37 ˚C, it unlocked the translation of GFP to highest yields. GJ2 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622010">BBa_K2622010</a>) was less leaky, but inhibited protein translation more strictly when grown at 37 ˚C. GJ6 (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622012">BBa_K2622012</a>), GJ9 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622013">BBa_K2622013</a>), and GJ10 (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622014">BBa_K2622014</a>) suppressed GFP production at 24 ˚C and 30 ˚C at similar level. They also inhibited translation to some extent at higher temperatures, meaning their melting temperature was not reached. Altogether these results prove, that our synthetic thermoswitches are temperature-responsive and act in physiological temperature range needed for IVTT reaction and also for BamA folding and membrane insertion. | pRSET plasmid and Sw<sub>x</sub> PCR products were digested with restriction enzymes and ligated, while GJ<sub>x</sub> PCR products were phosphorylated and ligated to produce plasmids from linear products. DH5α competent cells were transformed and plated on lysogeny broth (LB) media with ampicillin (Amp) and grown for 16 hours. Positive colonies were selected by colony PCR or restriction analysis (Fig. 3 and Fig. 4) and grown in 5 mL LB media. Plasmids were purified and BL21 competent cells were transformed. Three tubes of every construct plus plasmid with GFP without RNA thermometer were grown till OD<sub>600</sub> reached 0.4. Control samples were taken and protein expression was induced with Isopropyl β-D-1-thiogalactopyranoside (IPTG). One tube of every construct was grown in 24 ˚C, 30 ˚C, and 37 ˚C. Samples were taken after 1 and 2 hours. SDS-PAGE was run (for elaborate protocol see Notebook/<a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols">Protocols</a>). Fig. 5, Fig 6 and Fig. 7 depicts GFP expression at different temperatures. Although our RNA thermometers were designed to melt at 37 ˚C, some displayed leakiness to different extent. GJ3 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622011">BBa_K2622011</a>) RNA thermometer was the leakeast and allowed for GFP translation at lower temperatures. On the other hand, when grown at 37 ˚C, it unlocked the translation of GFP to highest yields. GJ2 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622010">BBa_K2622010</a>) was less leaky, but inhibited protein translation more strictly when grown at 37 ˚C. GJ6 (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622012">BBa_K2622012</a>), GJ9 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622013">BBa_K2622013</a>), and GJ10 (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622014">BBa_K2622014</a>) suppressed GFP production at 24 ˚C and 30 ˚C at similar level. They also inhibited translation to some extent at higher temperatures, meaning their melting temperature was not reached. Altogether these results prove, that our synthetic thermoswitches are temperature-responsive and act in physiological temperature range needed for IVTT reaction and also for BamA folding and membrane insertion. | ||
</p> | </p> | ||
| − | <p | + | <p> |
<img src="https://static.igem.org/mediawiki/2018/d/d8/T--Vilnius-Lithuania--THERMO_fig_3.png"/> | <img src="https://static.igem.org/mediawiki/2018/d/d8/T--Vilnius-Lithuania--THERMO_fig_3.png"/> | ||
<strong>Fig. 3</strong> Restriction analysis of GJ<sub>x</sub> constructs | <strong>Fig. 3</strong> Restriction analysis of GJ<sub>x</sub> constructs | ||
</p> | </p> | ||
| − | + | <p> | |
| − | <p | + | |
<img src="https://static.igem.org/mediawiki/2018/a/a4/T--Vilnius-Lithuania--THERMO_fig_4.png"/> | <img src="https://static.igem.org/mediawiki/2018/a/a4/T--Vilnius-Lithuania--THERMO_fig_4.png"/> | ||
<strong>Fig. 4</strong> Colony PCR of RNA thermometers in pSB1C3 plasmid. | <strong>Fig. 4</strong> Colony PCR of RNA thermometers in pSB1C3 plasmid. | ||
</p> | </p> | ||
| − | + | <p> | |
| − | <p | + | |
<img src="https://static.igem.org/mediawiki/2018/4/46/T--Vilnius-Lithuania--THERMO_fig_5.png"/> | <img src="https://static.igem.org/mediawiki/2018/4/46/T--Vilnius-Lithuania--THERMO_fig_5.png"/> | ||
<strong>Fig. 5</strong> expression at 24 ˚C. On the right you can see GFP expression without RNA thermometer. | <strong>Fig. 5</strong> expression at 24 ˚C. On the right you can see GFP expression without RNA thermometer. | ||
</p> | </p> | ||
| − | + | <p> | |
| − | <p | + | |
<img src="https://static.igem.org/mediawiki/2018/d/dd/T--Vilnius-Lithuania--THERMO_fig_6.png"/> | <img src="https://static.igem.org/mediawiki/2018/d/dd/T--Vilnius-Lithuania--THERMO_fig_6.png"/> | ||
<strong>Fig. 6</strong> GFP expression at 30 ˚C. On the right you can see GFP expression without RNA thermometer. | <strong>Fig. 6</strong> GFP expression at 30 ˚C. On the right you can see GFP expression without RNA thermometer. | ||
</p> | </p> | ||
| − | + | <p> | |
| − | <p | + | |
<img src="https://static.igem.org/mediawiki/2018/7/78/T--Vilnius-Lithuania--THERMO_fig_7.png"/> | <img src="https://static.igem.org/mediawiki/2018/7/78/T--Vilnius-Lithuania--THERMO_fig_7.png"/> | ||
<strong>Fig. 7</strong> GFP expression in 37 ˚C. On the right you can see GFP expression without RNA thermometer. | <strong>Fig. 7</strong> GFP expression in 37 ˚C. On the right you can see GFP expression without RNA thermometer. | ||
</p> | </p> | ||
| − | |||
<p></p> | <p></p> | ||
<h1>Discussion</h1> | <h1>Discussion</h1> | ||
| Line 261: | Line 255: | ||
As described in other sections of the Design and results page <a href="https://2018.igem.org/Team:Vilnius-Lithuania/Design"></a>, beta-barrel bearing proteins are assembled into the membrane by the BAM protein complex machinery. The key protein BamA is itself a membrane protein, whose folding and insertion into membrane where it helps assemble target proteins, last up to two hours. In order to prevent the aggregation of our fusion proteins after encapsulating their gene-bearing plasmids and purified BamA mRNA into liposomes, we needed to develop a modulatory regulatory tool to lock the translation of our membrane proteins to allow enough time for the encapsulated BamA to fold and insert into the liposome membrane. | As described in other sections of the Design and results page <a href="https://2018.igem.org/Team:Vilnius-Lithuania/Design"></a>, beta-barrel bearing proteins are assembled into the membrane by the BAM protein complex machinery. The key protein BamA is itself a membrane protein, whose folding and insertion into membrane where it helps assemble target proteins, last up to two hours. In order to prevent the aggregation of our fusion proteins after encapsulating their gene-bearing plasmids and purified BamA mRNA into liposomes, we needed to develop a modulatory regulatory tool to lock the translation of our membrane proteins to allow enough time for the encapsulated BamA to fold and insert into the liposome membrane. | ||
</p> | </p> | ||
| − | <p | + | <p> |
<img src="https://static.igem.org/mediawiki/2018/a/af/T--Vilnius-Lithuania--Fig8_NEW_thermoswitches.png"/> | <img src="https://static.igem.org/mediawiki/2018/a/af/T--Vilnius-Lithuania--Fig8_NEW_thermoswitches.png"/> | ||
<strong>Fig. 8</strong> Associational scheme of thermoswitches’ action in the SynDrop system. Not locking the concomitant translation of our target protein and BamA results in target protein aggregation due to insufficient membrane insertion and assembling potential of BamA. | <strong>Fig. 8</strong> Associational scheme of thermoswitches’ action in the SynDrop system. Not locking the concomitant translation of our target protein and BamA results in target protein aggregation due to insufficient membrane insertion and assembling potential of BamA. | ||
</p> | </p> | ||
| − | |||
<p> | <p> | ||
| − | |||
<img src="https://static.igem.org/mediawiki/2018/8/8b/T--Vilnius-Lithuania--Fig9_NEW_thermoswitches.png"/> | <img src="https://static.igem.org/mediawiki/2018/8/8b/T--Vilnius-Lithuania--Fig9_NEW_thermoswitches.png"/> | ||
<strong>Fig. 9</strong> Associational scheme of thermoswitches’ action in the SynDrop system. Locking up translation gives time for proper folding and insertion of BamA and prevents undesirable aggregation of target membrane proteins. | <strong>Fig. 9</strong> Associational scheme of thermoswitches’ action in the SynDrop system. Locking up translation gives time for proper folding and insertion of BamA and prevents undesirable aggregation of target membrane proteins. | ||
</p> | </p> | ||
| − | |||
<p> | <p> | ||
Additionally, while creating SynDrop, we have considered various options on how to make our complex cell-free system more user-controllable and predictable. Cell-free systems are becoming an attractive platform for <var>in vitro</var> compartmentalization and protein research, and although usually compositionally sensitive, they also offer a platform for building synthetic genetic regulatory tools or logic gates. Both the need to control the translation time of target genes and desire to provide more modularity for our synthetic system, led us to exploring RNA thermometers as a viable option to perform these tasks. They have minimal molecular burden and are easy to modulate. These properties encouraged us to developed a library of synthetic RNA thermometers suitable to translationally regulate the expression of our fusion constructs in bacteria with a further possibility to transfer them to IVTT systems. All of the RNA thermometers including those we found in literature and our <var>de novo</var> modelled ones were optimized for best performance at 37<sup>o</sup>C, bearing in mind their future transition to IVTT system, whose optimum performance temperature is also 37<sup>o</sup>C. Consequently, our experiments showed that our synthetic RNA thermometers, despite their simplistic structures compared to naturally occurring ones, efficiently triggered the expression of target constructs at 37<sup>o</sup>C, and successfully locked it at lower temperatures having made them an ideal complement to our liposome IVTT system. All of our thermoswitches unlocked the expression to similarly high levels at 37<sup>o</sup>C, but differed in terms of leakiness and success at inhibiting translation at lower temperatures. | Additionally, while creating SynDrop, we have considered various options on how to make our complex cell-free system more user-controllable and predictable. Cell-free systems are becoming an attractive platform for <var>in vitro</var> compartmentalization and protein research, and although usually compositionally sensitive, they also offer a platform for building synthetic genetic regulatory tools or logic gates. Both the need to control the translation time of target genes and desire to provide more modularity for our synthetic system, led us to exploring RNA thermometers as a viable option to perform these tasks. They have minimal molecular burden and are easy to modulate. These properties encouraged us to developed a library of synthetic RNA thermometers suitable to translationally regulate the expression of our fusion constructs in bacteria with a further possibility to transfer them to IVTT systems. All of the RNA thermometers including those we found in literature and our <var>de novo</var> modelled ones were optimized for best performance at 37<sup>o</sup>C, bearing in mind their future transition to IVTT system, whose optimum performance temperature is also 37<sup>o</sup>C. Consequently, our experiments showed that our synthetic RNA thermometers, despite their simplistic structures compared to naturally occurring ones, efficiently triggered the expression of target constructs at 37<sup>o</sup>C, and successfully locked it at lower temperatures having made them an ideal complement to our liposome IVTT system. All of our thermoswitches unlocked the expression to similarly high levels at 37<sup>o</sup>C, but differed in terms of leakiness and success at inhibiting translation at lower temperatures. | ||
Revision as of 23:47, 17 October 2018
Design and Results
Results
Cell-free, synthetic biology systems open new horizons in engineering biomolecular systems which feature complex, cell-like behaviors in the absence of living entities. Having no superior genetic control, user-controllable mechanisms to regulate gene expression are necessary to successfully operate these systems. We have created a small collection of synthetic RNA thermometers that enable temperature-dependent translation of membrane proteins, work well in cells and display great potential to be transferred to any in vitro protein synthesis system.


 Fig. 1 Principle of ribosome attachment to the liposome membrane. The ribosome exit tunnel is localized near the membrane, resulting in transmembrane domains of newly synthesized peptides interacting with the membrane, reducing aggregation
Fig. 1 Principle of ribosome attachment to the liposome membrane. The ribosome exit tunnel is localized near the membrane, resulting in transmembrane domains of newly synthesized peptides interacting with the membrane, reducing aggregation
 Fig. 2 Scheme of the genome modification process:
Fig. 2 Scheme of the genome modification process:
 Fig.3 Example of a constructed donor sequence. The sequence of the selected tag is present in primer used for the PCR of the homology arm that encompasses the target subunit. As a result, the tag sequence is fused to the ribosomal subunit gene.
Fig.3 Example of a constructed donor sequence. The sequence of the selected tag is present in primer used for the PCR of the homology arm that encompasses the target subunit. As a result, the tag sequence is fused to the ribosomal subunit gene.
 Fig. 4 PCR of homology arms, and antibiotic resistance genes
Fig. 4 PCR of homology arms, and antibiotic resistance genes
 Fig. 5 Constructed donor DNA sequences. The L29 donor DNA was not further revisited due to time constraints
Fig. 5 Constructed donor DNA sequences. The L29 donor DNA was not further revisited due to time constraints

 Fig. 2 Electrophoresis gel of PCR products: 6 - Sw2, 7 - Sw3, 8 - Sw6, 9 - Sw7, 10 - Sw9, 11 - Sw11.
Fig. 2 Electrophoresis gel of PCR products: 6 - Sw2, 7 - Sw3, 8 - Sw6, 9 - Sw7, 10 - Sw9, 11 - Sw11.
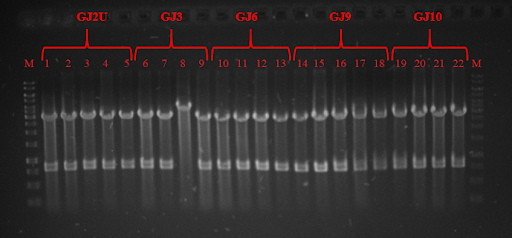 Fig. 3 Restriction analysis of GJx constructs
Fig. 3 Restriction analysis of GJx constructs
 Fig. 4 Colony PCR of RNA thermometers in pSB1C3 plasmid.
Fig. 4 Colony PCR of RNA thermometers in pSB1C3 plasmid.
 Fig. 5 expression at 24 ˚C. On the right you can see GFP expression without RNA thermometer.
Fig. 5 expression at 24 ˚C. On the right you can see GFP expression without RNA thermometer.
 Fig. 6 GFP expression at 30 ˚C. On the right you can see GFP expression without RNA thermometer.
Fig. 6 GFP expression at 30 ˚C. On the right you can see GFP expression without RNA thermometer.
 Fig. 7 GFP expression in 37 ˚C. On the right you can see GFP expression without RNA thermometer.
Fig. 7 GFP expression in 37 ˚C. On the right you can see GFP expression without RNA thermometer.
 Fig. 8 Associational scheme of thermoswitches’ action in the SynDrop system. Not locking the concomitant translation of our target protein and BamA results in target protein aggregation due to insufficient membrane insertion and assembling potential of BamA.
Fig. 8 Associational scheme of thermoswitches’ action in the SynDrop system. Not locking the concomitant translation of our target protein and BamA results in target protein aggregation due to insufficient membrane insertion and assembling potential of BamA.
 Fig. 9 Associational scheme of thermoswitches’ action in the SynDrop system. Locking up translation gives time for proper folding and insertion of BamA and prevents undesirable aggregation of target membrane proteins.
Fig. 9 Associational scheme of thermoswitches’ action in the SynDrop system. Locking up translation gives time for proper folding and insertion of BamA and prevents undesirable aggregation of target membrane proteins.




 Fig. 1 Simplified structure of scFv Antibody
Fig. 1 Simplified structure of scFv Antibody
 Fig. 2 Scheme of scFv_antiVLY and VLY interaction. Left- scFv_antiVLY binds to VLY, erythrocytes stay intact, Right- scFv_antiVLY does not bind and VLY lyse erythrocytes.
Fig. 2 Scheme of scFv_antiVLY and VLY interaction. Left- scFv_antiVLY binds to VLY, erythrocytes stay intact, Right- scFv_antiVLY does not bind and VLY lyse erythrocytes.
 Fig. 3 SDS-PAGE of scFv. GFP is used as positive control, C- chaperone DnaK.
Fig. 3 SDS-PAGE of scFv. GFP is used as positive control, C- chaperone DnaK.
 Fig. 4 Percentage of erythrocyte lysis at different +/-scFv dilutions.
Fig. 4 Percentage of erythrocyte lysis at different +/-scFv dilutions.
 Fig. 5 A- MstX-scFv_antiVLY expression in Escherichia coli. B- scFv_antiVLY and MstX-scFv_antiVLY expression in cell-free system.
Fig. 5 A- MstX-scFv_antiVLY expression in Escherichia coli. B- scFv_antiVLY and MstX-scFv_antiVLY expression in cell-free system.
 Fig. 6 Fig 6. Percentage of erythrocyte lysis at different scFv/MstX-scFv dilutions.
Fig. 6 Fig 6. Percentage of erythrocyte lysis at different scFv/MstX-scFv dilutions.