Phactory revolutionizes the production of therapeutic phages independently from their bacterial hosts. Each step of our phage production line was optimized individually to meet Good Manufacturing Practice and guarantee all safety requirements for therapeutic phages. Specifically engineered E. coli strains are the basis for the preparation of our home made cell extract to minimize remaining endotoxins and to maximize phage assembling quality. The simplicity of Phactory enables phage assembly by simply adding high-purity phage genomic DNA to the cell extract. This opens flexibel and individualized medical treatment of patients. Highest phage purity and high titers are ensured by further filtration and purification steps. Furthermore Phactory involves the packaging of our phages in alginate capsules for protection from gastric acid after oral administration and intestinal delivery.
Safety Requirements for Therapeutic Phages
 Identification
Identification
 Contamination
Contamination
 Toxicity
Toxicity
 pH
pH
 Purity
Purity
 Preservation
Preservation
High Quality Transcription-Translation Machinery
The preparation of home made cell extract for the production of therapeutics is very time intensive. For this reason we designed an msbB mutant strain to get steps for the elimination of endotoxin Lipid A off the protocol.

A LAL test was used to measure endotoxin values. We achieved a 49 fold lower Lipid A value in our home made cell extract compared to the commercialized myTX-TL.

We have approved, that the assembled phage solution has a physiological pH to ensure the requirement of the correct pH range for therapeutic phages.

Furthermore, we investigated different steps of the protocol to optimize cell extract quality concerning the following:
We compared the fluorescence time trace of a Malachite Green binding aptamer in different cell extract samples. The fluorescence time traces decline after 30-50min indicating, that RNA degradation starts to prevail over transcription. Differences in the observed kinetics can be explained by variations in cell extract composition.

Translation efficiency is five times higher than in commercialized TX/TL.
Preservation of Cell Extract

To make our cell extract accessible to everyone everywhere, we seeled to ensure long term storage and shipping at room temperature. Therefore we created lyophilized cell extract. The results show, that the tested samples retained 70% and 90% of expression quality respectively after lyophilization.
Furthermore, we tested the functionality of phages assembled in our home made cell extract after lyophilization and found functional phages afterwards.

Phage Assembly
The simplicity of Phactory offers the possibility of producing phages simply by adding the specific phage DNA to the TX-TL machinery. Moreover, Phactory yields excellent titers of assembled phages by far higher as required for therapeutic use.
Phactory makes high yield of phages of up to 1012 PFU/mL in a small reaction volume of 9 µL possible


The abscence of impurities in the phage solutions produced in Phactory was certified by Transmission Electron Microscopy (TEM) imaging.

Sequ-Into


For Phactory we intend to only use sequenced phage DNA to ensure the production of the volitional phage. We optimized the protocol for phage DNA purification to eliminate the possibility of generating a mix of phages unintentionally. Our software Sequ-Into was used for accurate quantification of DNA contamination levels. With our optimized purification methods we achieved a final DNA purity of 96 %. In comparison to that common phage DNA purification methods only achieve a purity of 65 %.

Purification
To fulfill the strict requirements for therapeutically used phages further purification is required to produce a high quality product.

By ultra-filtration phages got successfully separated from submolecular content.

Packaging
Phactory yields phages with toxicity levels that allow oral administration to the patient. However, oral delivery requires protection of the phages from rapid degradation in the acidic gastric juice. Therefore, we encapsulated our phages in alginate. This protects our phages successfully until release into the gastric system. We were able to prove functionality of encapsulated and released phages.



The Product
Phactory presents a complete production line from the optimized transcription-translation machinery as a platform for phage assembly to the packaging for final treatment. After all, our phages show excellent activity.
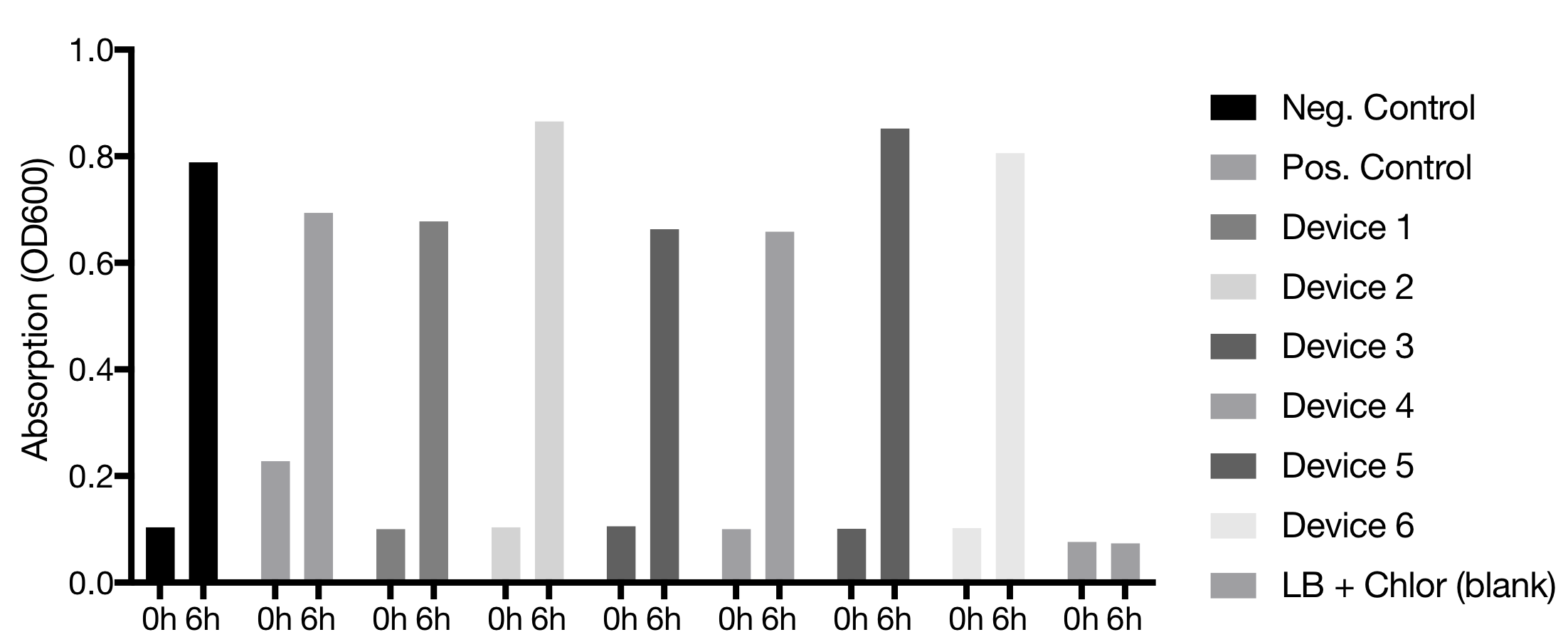
Plaque Assay of T7 phage over 6 hours
Clinically Relevant Bacteriophages
It was of great importance for us to demonstrate the medical relevance of bacteriophages manufactured with Phactory. We received the SEC-3S bacteriophage from the Queen Astrid Hospital as well as the P2 and P3 bacteriophages from the group of Laurent Debarbieux at the Pasteur Institute in Paris. These bacteriophages are specific for the E.Coli strain O104:H4, also known as EHEC, which caused 53 deaths during an outbreak in Germany in 2011. As indicated in the microscopy images, we successfully assembled these medically relevant bacteriophages in our cell-free system. We conducted a LAL-test and compared the results with magistral bacteriophage preparation regulations. According to these results, our bacteriophages are orally applicable. In the future we will work on reaching even higher titers with Phactory, so that we can apply more extensive purification protocols to allow intravenous application of bacteriophage therapy.




















