DEMONSTRATE
The integration of our entrepreneurial and social analysis led us to consider finding a way to functionalize bacterial cellulose. For this purpose, we designed a three headed proteic platform that we named Cerberus in reference to the mythological dog. It is composed of a cellulose-binding molecular module (CBM3a) fused at its N-terminal part to a biotinylated molecule-binding head (monomeric streptavidin) and at its C-terminal part to an unnatural amino acid (UnAA) azido-L-phenylalanine moiety. We also successfully set up a workflow allowing the synthesis of Cerberus (figure 1).


Validation of the Three Binding Heads
 Cellulose Binding
Cellulose Binding
We designed a fusion protein between the Carbohydrate Binding Module type 3a and a fluorescent reporter and named it Sirius as a reference to the brightest star of the norther hemisphere. Its purpose is to validate the association of our system towards cellulose. We demonstrated, using mRFP1 alone as negative control, the binding of our protein as showned in figure 2. We were then confident in the binding of our protein to cellulose.

 Biotinylated compound affinity
Biotinylated compound affinity
Our second construction consisted on our protein Cerberus but lacking the un natural amino acid. This construction aimed to prove the binding affinity of our system toward biotinylated compounds. As biotinylation can be both performed at high yield in vivo and in vitro, this construction is already a molecular binding plateform, allowing the purification of biotinylated compounds at low cost, using cellulose. We validated this construction using biotinylated mTag Blue Fluorescent Protein (figure3.a), biotinylated in vivo in E. coli and Scygonadin, an anti microbial peptide, produced in Pichia pastoris (figure 3.b).

 Click chemistry
Click chemistry
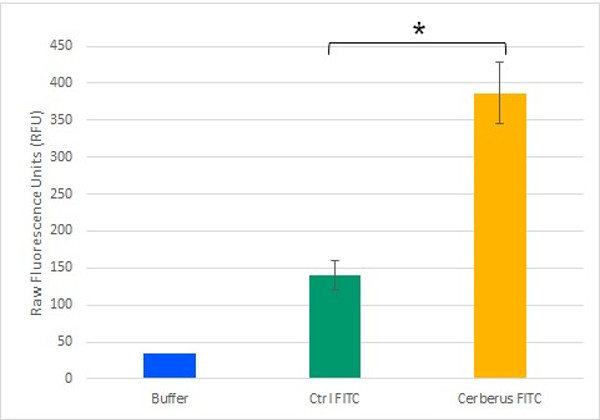
Our plateform aims to have a binding capacity as wide as possible. The azide groupment offer a high efficiency covalent bound formation through cycloadditionreaction, mostyl known by Click chemistry. The possibilities offered by this system are therefore included in the unnatural amino acid : 4-azido-L-phenylalanine. We designed our protein to bear an Amber stop codon for unnatural amino acid integration in our plateform. We confirmed the activity of this integration using DBCO-Fluorescein as showed in figure 4. We also functionnalized cellulose using paramagnetic beads (see video below) which shows the broad range functionnalization possibilities that our system offers.
Compounds Fuctionalization
Production and Functionalization of Bacterial Cellulose


Conclusion
No dogs were harmed over the course of this iGEM project.
The whole Toulouse INSA-UPS team wants to thank our sponsors, especially:









And many more. For futher information about our sponsors, please consult our Sponsors page.
The content provided on this website is the fruit of the work of the Toulouse INSA-UPS iGEM Team. As a deliverable for the iGEM Competition, it falls under the Creative Commons Attribution 4.0. Thus, all content on this wiki is available under the Creative Commons Attribution 4.0 license (or any later version). For futher information, please consult the official website of Creative Commons.
This website was designed with Bootstrap (4.1.3). Bootstrap is a front-end library of component for html, css and javascript. It relies on both Popper and jQuery. For further information, please consult the official website of Bootstrap.



