Our Design
Overview
We decided to develop a probiotic strain of E. coli as a novel, self-tuning therapeutic for IBD. Our core design consists of the following:
- NO-dependent IL-10 secretion system
- Membrane-anchored nucleoside hydrolase
- Riboswitch-ribozyme-sRNA construct
- Inducible kill switch
This provides a framework that can be used in a huge range of new projects by future iGEM teams, including biosensors and therapeutics.
Due to the size and impermeability of regulatory interleukin proteins to the bacterial cell membrane, we used adenosine and nitric oxide (NO) as metabolic markers of Treg and Th-17 function respectively. An imbalance in the levels correlates with the autoimmune response. We aim to restore these to healthy proportions by secreting Interleukin 10 (IL-10) - a signal protein which stimulates cell differentiation into T-reg cells.
IL-10 expression is stimulated by the endogenous E. coli SoxR transcription factor, activated by free radicals and oxidative stress, while expression is inhibited in response to adenosine by means of an adenine transcriptional riboswitch linked to sRNA synthesis which will selectively inhibit translation of IL-10 mRNA. The localised action of IL-10 secreted from the engineered bacteria makes our therapeutic best suited to gastrointestinal-based autoimmune diseases. Thus, we decided to focus specifically on IBD.
A single stimulus can result in false positives and excessive suppression, leading to immunodeficiency. Incorporation of a negative feedback loop signaling high Treg populations avoids oversuppression of the immune system. The defining aspect of our design is the integration of two signals in order to increase the specificity and accuracy of our system in equilibrating the populations of Treg and Th-17 cells.

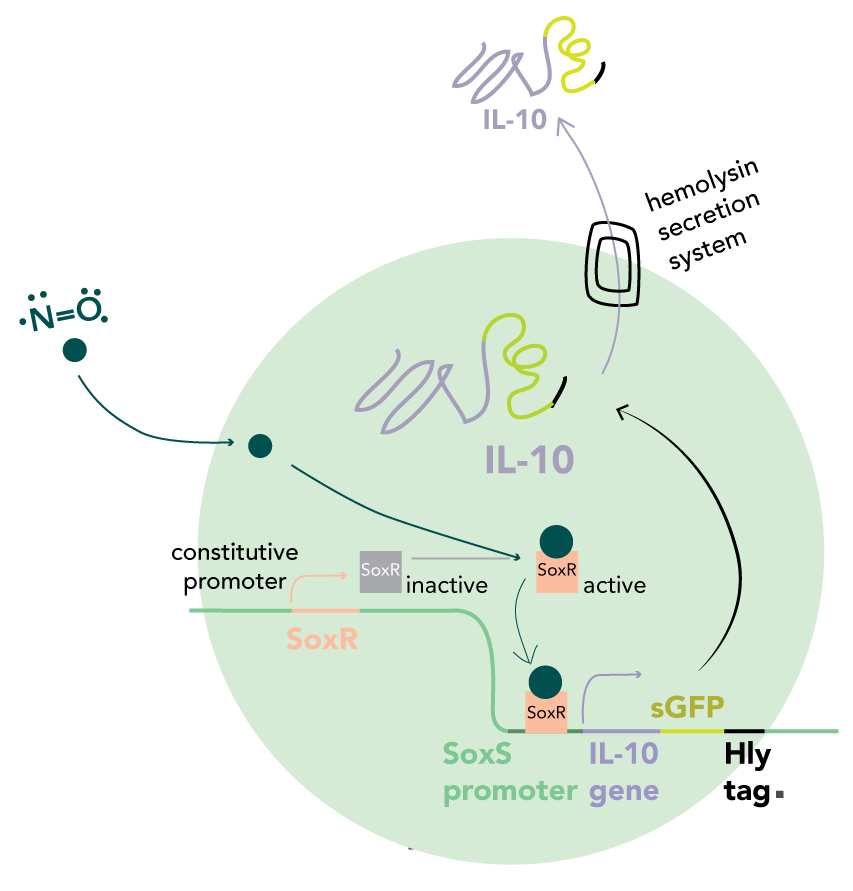
NO-dependent IL-10 secretion system
In our circuit, IL-10 is secreted in the presence of NO and absence of elevated extracellular adenosine. We decided to use NO partly due to the work of the Stanford 2009 iGEM team, who used NO detection to activate the synthesis of retinoic acid. We used the same SoxR/SoxS promoter system to detect NO but instead, are using it to stimulate IL-10 production.
The SoxS promoter (pSoxS) is an oxidative stress-responsive element, activated by the dimeric transcription factor, SoxR. Oxidation of the iron-sulphur cluster by NO leads to a shift into the active state.
IL-10 is targeted for secretion by fusion with a C-terminal HlyA tag. This is recognised by the HlyD/HlyB/TolC complex which constitutes the type I secretory apparatus. By combining the four genes into a single operon under control of the SoxS promoter, both IL-10 production and its export can be upregulated in response to an elevated NO concentration. Therefore, transcription of new mRNA and export of previously synthesised proteins are inhibited in the absence of an inducer.
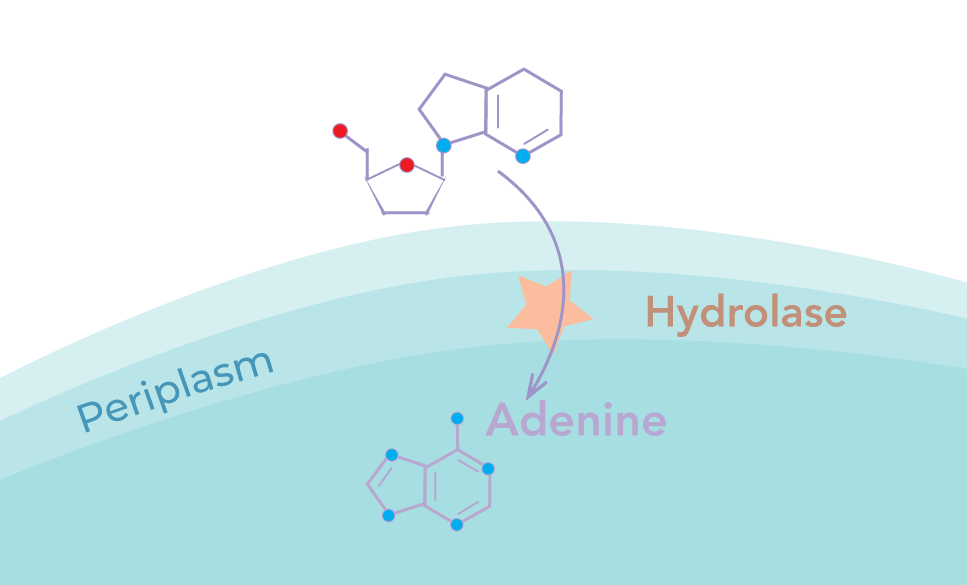
Membrane-anchored nucleoside hydrolase
We implemented a membrane-anchored adenosine hydrolase to catalyse the breakdown of extracellular adenosine into adenine. This would then diffuse into the cytoplasm and control the negative feedback loop
The hydrolase was designed to be excluded from the cytoplasm in order to maximise the signal to noise ratio. This is particularly necessary due to the action of periplasmic deaminase in converting adenosine to inosine, which cannot be detected using a riboswitch. Cytoplasmic adenosine, if hydrolysed, would lead to alteration of metabolic function, resulting in a high intracellular adenine level independent of extracellular adenosine. Secretion across the inner membrane thus increases the sensitivity of the response whilst minimising the impact of our device on the growth of the chassis.
Two independent parts were designed to fulfil this role:
Periplasmic Hydrolase
The type II system, TAT, is well characterised, allowing the secretion of protein complexes into the periplasm. By attaching a C-terminal secretion tag, the hydrolase dimer can be secreted in a folded state.
Membrane anchored hydrolase
Fusion of the hydrolase to a truncated form of the YiaT membrane protein using a flexible linker was designed to promote hydrolase localisation for surface expression. Given that dimer formation is believed to be critical for enzyme activity and the YiaT transport system has not been shown to co-transport bound proteins across the membrane, a linker of increased length was used to join two hydrolase monomers together, forming a concatemer with dimerisation capabilities.
Riboswitch-ribozyme-sRNA construct
PbuE Riboswitch
While adenosine binds to no known transcriptional regulator in bacteria, it can be detected using its hydrolytic product, adenine, which binds to the PbuE riboswitch as an indirect measure of concentration. PbuE is a transcriptional activating riboswitch, thought to function by the unfolding of a terminator loop in response to ligand binding to the aptamer domain.
An upstream non-coding region was inserted before the aptamer which has been shown to improve folding efficiency. In order to investigate the importance of the upstream region, we designed two different riboswitch variants:
- PbuE riboswitch with 89 bp upstream region
- PbuE riboswitch with 117 bp upstream region
sRNA
In the system, sRNA is a critical feature, converting the adenosine input into repression of IL-10 translation. The sRNA contains a sequence, complementary to the IL-10 mRNA, of variable length, allowing tuning of binding affinity to alter sensitivity to the adenosine levels.
A MicC scaffold in the sRNA recruits an mRNA degradation complex.
Ribozyme
sRNA has been shown to only exhibit knockdown when not connected to auxiliary RNA such as mRNA. Cleavage from the riboswitch, to activate the sRNA, is performed using a ribozyme. The hepatitis delta virus ribozyme (HDV) was chosen over the better characterised human hammerhead ribozyme given that the latter included a PstI site which is incompatible with the BioBrick standards.

Inducible kill switch
Another important consideration was to develop a system to enhance the safety of our probiotic. Therefore, we decided to design a kill switch which would be activated by an external supplement in order to account for the possibility of adverse reactions in patients. We linked the antimicrobial artilysin Art-175 with a DsbA periplasmic secretion tag, under the control of an inducible pTet promoter. Therefore, upon induction by a synthetic TetR inducer, expression of the artilysin composite would promote host cell lysis. Two variants were designed:
- Artilysin-DsbA controlled by unidirectional pTet
- Artilysin-DsbA controlled by bidirectional pTet
The development of a bidirectional variant was key for implementing the kill switch in bacterial strains that do not endogenously produce TetR. Having established the versatility of this design, we decided to introduce the bidirectional pTet promoter to the registry for use by future iGEM teams in a wide range of applications.

