Kasparas12 (Talk | contribs) |
|||
| (18 intermediate revisions by 3 users not shown) | |||
| Line 22: | Line 22: | ||
<div class="modal-close"></div> | <div class="modal-close"></div> | ||
<div class="modal-content"> | <div class="modal-content"> | ||
| − | + | <section class="design_subsections"> | |
| − | + | <h1 id="Liposomes">Liposomes</h1> | |
| − | + | <div class="third_level_links"> | |
<a href="#Liposomes">Liposomes</a> | <a href="#Liposomes">Liposomes</a> | ||
<a href="#Ribosome_modifications">Ribosome modifications</a> | <a href="#Ribosome_modifications">Ribosome modifications</a> | ||
| Line 36: | Line 36: | ||
<p></p> | <p></p> | ||
<p> | <p> | ||
| − | At the core of SynDrop lays a liposome. Liposomes are essentially synthetic vesicles, artificially synthesized droplets of liquid, separated from the environment by a lipid bilayer (Fig.1). They act as containers that encapsulate purified transcriptional and translational machinery and other vital elements that enable complex circuitry design. They have become increasingly popular due to various applications such as being carriers for medicinal drugs<sup>1</sup>, closed environments for protein engineering<sup>2</sup> and characterization of RNAs<sup>3</sup>, as biosensors<sup>4</sup> and molecular diagnostic tools<sup>5</sup>. The growing perspectives of liposomes as scaffolds for synthetic circuitry and membrane protein research are compelling as they have a multitude of different parameters that can be controlled. These include size, composition of a lipid membrane and interior composition. | + | At the core of SynDrop lays a liposome. Liposomes are essentially synthetic vesicles, artificially synthesized droplets of liquid, separated from the environment by a lipid bilayer (Fig. 1). They act as containers that encapsulate purified transcriptional and translational machinery and other vital elements that enable complex circuitry design. They have become increasingly popular due to various applications such as being carriers for medicinal drugs<sup>1</sup>, closed environments for protein engineering<sup>2</sup> and characterization of RNAs<sup>3</sup>, as biosensors<sup>4</sup> and molecular diagnostic tools<sup>5</sup>. The growing perspectives of liposomes as scaffolds for synthetic circuitry and membrane protein research are compelling as they have a multitude of different parameters that can be controlled. These include size, composition of a lipid membrane and interior composition. |
</p> | </p> | ||
<p> | <p> | ||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/c/cc/T--Vilnius-Lithuania--Fig_1_NEW_su_uzrasu_Liposomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/c/cc/T--Vilnius-Lithuania--Fig_1_NEW_su_uzrasu_Liposomes.png"/> | ||
| − | <p><strong>Fig 1</strong> The composition of a liposome with encapsulated machinery for membrane protein integration. Size, membrane composition and interior composition can be easily varied.</p> | + | <p><strong>Fig. 1</strong> The composition of a liposome with encapsulated machinery for membrane protein integration. Size, membrane composition and interior composition can be easily varied.</p> |
</p> | </p> | ||
</div> | </div> | ||
| − | + | <h2>Requirements for liposomes</h2> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<p></p> | <p></p> | ||
<p> | <p> | ||
| Line 64: | Line 60: | ||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/0/0e/T--Vilnius-Lithuania--Fig2_Liposomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/0/0e/T--Vilnius-Lithuania--Fig2_Liposomes.png"/> | ||
| − | <p><strong>Fig 2 a </strong>AutoCAD design for the photomask. There are 16 individual microchannel devices on a | + | <p><strong>Fig. 2 a </strong>AutoCAD design for the photomask. There are 16 individual microchannel devices on a |
| − | + | ||
| + | single chip. <strong>b</strong> One device consists of three inlets, an outlet and a star-shaped junction.</p> | ||
</p> | </p> | ||
</div> | </div> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<h2>Photolithography as a tool for microfluidic chip fabrication</h2> | <h2>Photolithography as a tool for microfluidic chip fabrication</h2> | ||
<p></p> | <p></p> | ||
| Line 82: | Line 70: | ||
After calculating the exact parameters for microfluidic channels and receiving a printed photomask, photolithography is performed to create a master for microfluidic chip preparation. After completing this step, PDMS (<var>polydimethylsiloxane</var>) is poured on to the master left in a thermostat overnight. Inlets and outlets are punched with a biopsy puncher, and the PDMS is cleaned and plasma treated before attaching it to the PDMS coated microscope glass slides. Fig. 3 presents a simplified scheme demonstrating photolithography and other +6steps towards creating a microfluidic chip. To learn more details about the fabrication process, refer to <a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols">our Protocols</a>. We called our chip LipoDrop. The final form of LipoDrop is shown in Fig. 4. | After calculating the exact parameters for microfluidic channels and receiving a printed photomask, photolithography is performed to create a master for microfluidic chip preparation. After completing this step, PDMS (<var>polydimethylsiloxane</var>) is poured on to the master left in a thermostat overnight. Inlets and outlets are punched with a biopsy puncher, and the PDMS is cleaned and plasma treated before attaching it to the PDMS coated microscope glass slides. Fig. 3 presents a simplified scheme demonstrating photolithography and other +6steps towards creating a microfluidic chip. To learn more details about the fabrication process, refer to <a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols">our Protocols</a>. We called our chip LipoDrop. The final form of LipoDrop is shown in Fig. 4. | ||
</p> | </p> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/7/7d/T--Vilnius-Lithuania--Fig3_Liposomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/7/7d/T--Vilnius-Lithuania--Fig3_Liposomes.png"/> | ||
| − | <p><strong>Fig 3 </strong> Simplified scheme for microfluidic device preparation. <strong>a-b</strong> the silicon wafer is cleaned and spin-coated with photoresist; <strong>c</strong> the photomask is aligned on the sample and exposed to UV light. <strong>d</strong> sample is submerged to a developer – only the sections that were exposed to the UV light remain intact on the wafer; <strong>e</strong> PDMS is poured onto the master to create a PDMS mold and left for a bake in the oven; <strong>f</strong> the mold is then separated and prepared further by cleaning and punching inlets and outlets; <strong>e-f</strong> a microscopic slide is prepared by applying a thin layer of PDMS on top; <strong>i</strong> PDMS mold and PDMS covered microscopic slide are plasma treated and connected to each other to produce a final microfluidic chip.</ | + | <p><strong>Fig. 3 </strong> Simplified scheme for microfluidic device preparation. <strong>a-b</strong> the silicon wafer is cleaned and spin-coated with photoresist; <strong>c</strong> the photomask is aligned on the sample and exposed to UV light. <strong>d</strong> sample is submerged to a developer – only the sections that were exposed to the UV light remain intact on the wafer; <strong>e</strong> PDMS is poured onto the master to create a PDMS mold and left for a bake in the oven; <strong>f</strong> the mold is then separated and prepared further by cleaning and punching inlets and outlets; <strong>e-f</strong> a microscopic slide is prepared by applying a thin layer of PDMS on top; <strong>i</strong> PDMS mold and PDMS covered microscopic slide are plasma treated and connected to each other to produce a final microfluidic chip.</div></p> |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/7/75/T--Vilnius-Lithuania--Fig4_Liposomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/7/75/T--Vilnius-Lithuania--Fig4_Liposomes.png"/> | ||
| − | <p><strong>Fig 4 </strong> Final form of Lipodrop. | + | <p><strong>Fig. 4 </strong> Final form of Lipodrop.</div> |
| − | + | ||
| − | + | ||
| − | + | ||
</p> | </p> | ||
| − | |||
<h2>Coating LipoDrop with PVA</h2> | <h2>Coating LipoDrop with PVA</h2> | ||
<p></p> | <p></p> | ||
| Line 108: | Line 84: | ||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/2/29/T--Vilnius-Lithuania--Fig5_Liposomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/2/29/T--Vilnius-Lithuania--Fig5_Liposomes.png"/> | ||
| − | <p><strong>Fig 5 </strong> A schematic representation of the interphase of air and PVA at the star shaped junction of LipoDrop.</p> | + | <p><strong>Fig. 5 </strong> A schematic representation of the interphase of air and PVA at the star shaped junction of LipoDrop.</p> |
</p> | </p> | ||
</div> | </div> | ||
| − | |||
<h2>Lipovision software for fully automated microfluidic experiments</h2> | <h2>Lipovision software for fully automated microfluidic experiments</h2> | ||
<p></p> | <p></p> | ||
| Line 125: | Line 100: | ||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/7/77/T--Vilnius-Lithuania--Fig6_Liposomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/7/77/T--Vilnius-Lithuania--Fig6_Liposomes.png"/> | ||
| − | <p><strong>Fig 6</strong> A close-up of the phase interface during liposome synthesis; <strong>IA</strong> phase contains elements required for the synthesis | + | <p><strong>Fig. 6</strong> A close-up of the phase interface during liposome synthesis; <strong>IA</strong> phase contains elements required for the synthesis |
and integration of membrane proteins; <strong>LO</strong> phase consists of octanol and lipids that form a lipid bilayer; OA solution | and integration of membrane proteins; <strong>LO</strong> phase consists of octanol and lipids that form a lipid bilayer; OA solution | ||
| − | carries surfactants that stabilize the initial formation and propagation of the droplets along the microfluidic device.</p> | + | carries surfactants that stabilize the initial formation and propagation of the droplets along the microfluidic device.</p></div> |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
<h2>Optimized flow rates for high throughput synthesis</h2> | <h2>Optimized flow rates for high throughput synthesis</h2> | ||
<p></p> | <p></p> | ||
| Line 142: | Line 113: | ||
</video> | </video> | ||
| − | <p><strong>Fig 7 </strong> High throughput formation of cell-sized liposomes. The video is 60x slowed down </p> | + | <p><strong>Fig. 7 </strong> High throughput formation of cell-sized liposomes. The video is 60x slowed down </p> |
</p> | </p> | ||
</div> | </div> | ||
| − | |||
| − | |||
| − | |||
A simple liposome size frequency distribution was determined with an image analysis software ImageJ. A plugin SpotCaliper was utilized to identify circular objects and measure their diameters (Fig. 8a). Gaussian distribution was fitted to the frequency histogram. Results verify that the size of the liposomes follows the Gaussian distribution (Fig. 8b). It proves that the droplets are highly homogeneous. Average diameter of a liposome (results from a single batch experiment) is around 12 µm, with standard deviation of 0.4 µm which fits our requirements very well. | A simple liposome size frequency distribution was determined with an image analysis software ImageJ. A plugin SpotCaliper was utilized to identify circular objects and measure their diameters (Fig. 8a). Gaussian distribution was fitted to the frequency histogram. Results verify that the size of the liposomes follows the Gaussian distribution (Fig. 8b). It proves that the droplets are highly homogeneous. Average diameter of a liposome (results from a single batch experiment) is around 12 µm, with standard deviation of 0.4 µm which fits our requirements very well. | ||
</p> | </p> | ||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/4/4e/T--Vilnius-Lithuania--Fig8_Liposomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/4/4e/T--Vilnius-Lithuania--Fig8_Liposomes.png"/> | ||
| − | <p><strong>Fig 8 </strong> An automatic detection of droplets with SpotCaliper: the droplets are marked with teal colored circles and the | + | <p><strong>Fig. 8 </strong> An automatic detection of droplets with SpotCaliper: the droplets are marked with teal colored circles and the |
diameter of each is measured; <strong>b</strong> size frequency distribution histogram fitted to Gaussian distribution (teal fit) proves | diameter of each is measured; <strong>b</strong> size frequency distribution histogram fitted to Gaussian distribution (teal fit) proves | ||
the homogeneity of the liposomes; μ=11.853 >µm±0.017 >µm ; SD=0.442 µm ±0.017 µm. <p></p> | the homogeneity of the liposomes; μ=11.853 >µm±0.017 >µm ; SD=0.442 µm ±0.017 µm. <p></p> | ||
| Line 159: | Line 127: | ||
</div> | </div> | ||
</p> | </p> | ||
| − | |||
<h2>Characterization: encapsulation efficiency and internal synthesis</h2> | <h2>Characterization: encapsulation efficiency and internal synthesis</h2> | ||
<p></p> | <p></p> | ||
| Line 170: | Line 137: | ||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/c/c2/T--Vilnius-Lithuania--Fig9_Liposomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/c/c2/T--Vilnius-Lithuania--Fig9_Liposomes.png"/> | ||
| − | <p><strong>Fig 9 </strong> brightfield image of the liposomes that contain IVTT system and plasmid GFP DNA (after incubation); | + | <p><strong>Fig. 9 </strong> brightfield image of the liposomes that contain IVTT system and plasmid GFP DNA (after incubation); |
scale bar is 10 µm; <strong>b</strong> liposomes imaged with FITC: fluorescence confirms that transcription and translation | scale bar is 10 µm; <strong>b</strong> liposomes imaged with FITC: fluorescence confirms that transcription and translation | ||
reactions occur inside them; scale bar is 10 µm; <strong>c</strong> liposomes containing purified GFP protein: all the | reactions occur inside them; scale bar is 10 µm; <strong>c</strong> liposomes containing purified GFP protein: all the | ||
| Line 178: | Line 145: | ||
</p> | </p> | ||
| − | |||
<h2>Characterization: unilamellarity validation using α-hemolysin protein pores</h2> | <h2>Characterization: unilamellarity validation using α-hemolysin protein pores</h2> | ||
<p></p> | <p></p> | ||
| Line 193: | Line 159: | ||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/3/34/T--Vilnius-Lithuania--Fig10_Liposomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/3/34/T--Vilnius-Lithuania--Fig10_Liposomes.png"/> | ||
| − | <p><strong>Fig 10 a</strong> concentrated calcein encapsulated within liposomes: the outer solution fluoresces as some of the liposomes | + | <p><strong>Fig. 10 a</strong> concentrated calcein encapsulated within liposomes: the outer solution fluoresces as some of the liposomes |
inevitably burst releasing calcein into the outside; <strong>b</strong> box plot comparison of the control (without α-hemolysin) and | inevitably burst releasing calcein into the outside; <strong>b</strong> box plot comparison of the control (without α-hemolysin) and | ||
a group with inserted α-hemolysin; nonparametrical Mann-Whitney U test was used for the statistical evaluation: | a group with inserted α-hemolysin; nonparametrical Mann-Whitney U test was used for the statistical evaluation: | ||
| Line 201: | Line 167: | ||
</p> | </p> | ||
| − | |||
<h3>References</h3> | <h3>References</h3> | ||
<p> | <p> | ||
| Line 303: | Line 268: | ||
</li> | </li> | ||
</ol></p> | </ol></p> | ||
| − | + | ||
| − | + | ||
</div> | </div> | ||
| − | For multi-gene editing, we chose to supply the donor sequence as a linear DNA strand (PCR product). Due to financial reasons, to construct the donor DNA sequence we performed separate PCRs of the homology arms (from the E. coli genome), selection marker (antibiotic resistance genes from available plasmids) (Fig. 4). The oligomers had the his and strep tag sequences incorporated into them alongside 2 different restriction sites. In case the distance between the ribosomes and the membrane wall was too small for our system to be efficient, we also designed alternative variants the would feature the his-tags connected via a highly flexible two-glycine-four-serine linker (GGSSSS), which is a highly popular linker for artificial fusion proteins. | + | <p>For multi-gene editing, we chose to supply the donor sequence as a linear DNA strand (PCR product). Due to financial reasons, to construct the donor DNA sequence we performed separate PCRs of the homology arms (from the E. coli genome), selection marker (antibiotic resistance genes from available plasmids) (Fig. 4). The oligomers had the his and strep tag sequences incorporated into them alongside 2 different restriction sites. In case the distance between the ribosomes and the membrane wall was too small for our system to be efficient, we also designed alternative variants the would feature the his-tags connected via a highly flexible two-glycine-four-serine linker (GGSSSS), which is a highly popular linker for artificial fusion proteins. |
</p> | </p> | ||
<p> | <p> | ||
| Line 321: | Line 285: | ||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/d/d5/T--Vilnius-Lithuania--Fig4_Ribosomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/d/d5/T--Vilnius-Lithuania--Fig4_Ribosomes.png"/> | ||
| − | <p><strong> Fig. 4 </strong> PCR of homology arms, and antibiotic resistance genes</p> | + | <p><strong>Fig. 4 </strong> PCR of homology arms, and antibiotic resistance genes</p> |
</p> | </p> | ||
</div> | </div> | ||
| Line 327: | Line 291: | ||
<div class="image-container"> | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/8/86/T--Vilnius-Lithuania--Fig5_Ribosomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/8/86/T--Vilnius-Lithuania--Fig5_Ribosomes.png"/> | ||
| − | <p><strong> Fig. 5 </strong> Constructed donor DNA sequences. The L29 donor DNA was not further revisited due to time constraints</p> | + | <p><strong>Fig. 5 </strong> Constructed donor DNA sequences. The L29 donor DNA was not further revisited due to time constraints</p> |
</p> | </p> | ||
</div> | </div> | ||
<p> | <p> | ||
| − | The genome modifications were then carried according to <a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols"> | + | The genome modifications were then carried according to <a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols">our protocol</a>. Although cPCR gave us mixed results, we could not verify any colonies that afterwards grew on our selected marker antibiotics, and thus could not continue our experiments with them. It appears most likely that the genome modifications were not entirely successful, due to the somewhat unstable nature of the ligated linear DNA used for the donor sequence. |
</p> | </p> | ||
<p></p> | <p></p> | ||
| Line 374: | Line 338: | ||
</div> | </div> | ||
<div> | <div> | ||
| − | + | <h1>Background</h1> | |
| − | + | <p> | |
| − | + | </p> | |
| − | + | <P>Proteins that belong to a small group referred to as nonconstitutive membrane proteins can independently integrate into the membrane from the aqueous phase without the help of other proteins. This is because they possess a stable soluble form, that can bind to membranes and then insert and refold into another stable form.[1] However, most of the membrane proteins do not have a stable soluble form and rapidly aggregate when synthesized in the cytoplasm. That is why living organisms require additional machinery which facilitates MP insertion into membranes and catalyzes their folding.</P> | |
| − | + | ||
| − | + | <h2>Structure of BAM complex | |
| − | + | </h2> | |
| − | + | <p>Assembly of the β-barrel bearing integral membrane proteins (MPs) into the target membrane is catalyzed by the β-barrel assembly machinery (BAM) complex. It contains five subunits, BamA–E.[2] BamA is composed of a 16-strand β-barrel integral membrane part and a periplasmic domain, which consists of five globular subdomains called POTRA motifs that are essential for complex formation and interaction with a substrate β-barrel proteins.[3] BamB-E are lipoproteins, each attaching to the inner leaflet of the OM via an N-terminal lipid moiety and playing an important role in promoting the folding of OMP.[4] (Fig.1) | |
| − | + | </p> | |
| − | + | <div class="image-container"> | |
| + | <img src="https://static.igem.org/mediawiki/2018/1/1c/T--Vilnius-Lithuania--Fig1_BAM_compl.png"/> | ||
| + | <p><strong>Fig. 1</strong> Structure of BAM complex</p> | ||
| + | </div> | ||
| + | <h2>Outer membrane protein (OMP) insertion into membrane in bacteria cells | ||
| + | </h2> | ||
| + | <div class="image-container"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/3/3a/T--Vilnius-Lithuania--Fig2_BAM_compl.png"/> | ||
| + | <p><strong>Fig. 2</strong> 5 steps scheme of OMP insertion into OM.</p> | ||
| + | </div> | ||
| + | |||
| + | <p>Generally MPs are integrated during translation with the assistance of the Sec Translocon. However, as there is no protein translation within the periplasm, OMPs require an alternative integration mechanism. | ||
| + | </p> | ||
| + | <p>In the beginning, OMPs are synthesized in the cytoplasm with N-terminal signal sequence that directs them to the Sec translocon, which transfers OMPs through the inner membrane into periplasm.[5] (Fig.2, step 1) | ||
| + | </p> | ||
| + | <p>Integral membrane proteins forming β-barrel structures are prone to aggregate in aqueous environments. Therefore, after they transit a Sec channel, chaperones are required to bind OMPs to transfer them through the periplasmic compartment, while keeping them in an unfolded state to prevent aggregation.(Fig.2, step 2) The periplasmic chaperone, SurA has been shown to transfer most of the OMPs to the OM.[6] It has been shown that SurA directly participates in Bam-mediated OMP assembly by associating with BamA POTRA domain.[7] (Fig.2, step 3)</p> | ||
| + | <p>Final folding takes place in BAM complex. However, the mechanism how BAM complex catalyzes the insertion of β-barrel proteins into the OM still remains not fully understood. Structure analysis implies that the cavity seems to be too small to accomodate a fully folded OMP substrate, even though it is large enough to house couple of substrate β-hairpins.[2] (Fig.2, step 4)Based on the structure, it has been suggested that the rotation of the ring-like structure by POTRA domains and lipoproteins leads to the opening of a junction between the first and last β-strands of the BamA β-barrel (lateral gate) which promotes the insertion of OMPs into the lipid bilayer.[8] (Fig. 2, step 5) | ||
| + | </p> | ||
| + | <h2>Our approach</h2> | ||
| + | <p>In order to reconstitute the fully working Bam complex we relied on the mechanisms elucidated before. SurA is a periplasmic chaperone which binds to unfolded β-barrel proteins and retains them in unfolded state, thus preventing aggregation. BamB or BamD lipoproteins can bind BamA-SurA, direct the complex to the membrane, and catalyze BamA insertion as well as correct folding in the membrane. [9] (Fig. 3, step 1) Knowing that in bacteria most of the Bam lipoproteins are found in BAM complex and full five proteins complex can be purified with one tag without any cross-links, we expected high complex association constants among the components (Fig.3, step 2) and hypothesized that BAM complex could be assembled in vitro simply by encapsulating BamA-SurA associatives and Bam lipoproteins.</p> | ||
| + | <div class="image-container"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/3/30/T--Vilnius-Lithuania--Fig3_BAM_compl.png"/> | ||
| + | <p><strong>Fig. 3</strong> Bam lipoproteins assemble BamA in vitro</p> | ||
| + | </div> | ||
| + | <p></p> | ||
| + | <h1>Results</h1> | ||
| + | <h2>Plasmid construction</h2> | ||
| + | <p>To purify the components of BAM complex we have constructed 6 plasmids:</p> | ||
| + | <p> | ||
| + | <ol> | ||
| + | <li>pET28b-BamA</li> | ||
| + | <li>pET22b-BamA</li> | ||
| + | <li>pET22b-BamB</li> | ||
| + | <li>pCDFDuet-BamC-BamD</li> | ||
| + | <li>pET22b-BamE</li> | ||
| + | <Li>pET28b-SurA(more details in protocols)</Li> | ||
| + | </ol> | ||
| + | which encode products of BamA-HisN6, BamA, BamB-HisC6, BamC, BamD, BamE-HisC8, SurA-HisN6. (Fig. 4 and Fig. 5) | ||
| + | </p> | ||
| + | <div class="image-container"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/5/54/T--Vilnius-Lithuania--Fig4.1_BAM_compl.png"/> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/3/3d/T--Vilnius-Lithuania--Fig4.2_BAM_compl.png"/> | ||
| + | <p><strong>Fig. 4</strong> Maps of constructed plasmids</p> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/2/2e/T--Vilnius-Lithuania--Fig5_BAM_compl.png"/> | ||
| + | <p><strong>Fig. 5</strong> PCR products of genes of BAM complex 1,18 – GeneRuler 1 kb DNA ladder; 4,5 – SurA (1250bp); 6,7 - BamE (390bp); 8,9 – BamB (1201bp); 10, 11 – BamC (1056bp); 12, 13 – BamD(756bp); 14,16 – BamA (2455bp)</p> | ||
| + | </div> | ||
| + | <h2> | ||
| + | Protein purification | ||
| + | </h2> | ||
| + | <p>We relied on three different strategies to purify the separate BAM complex components: </p> | ||
| + | <ul> | ||
| + | <li>For our experiments we needed an unfolded BamA. Therefore, we overexpressed BamA , which we isolated in the form of inclusion bodies and then dissolved in 8M urea without any further purification steps.</li> | ||
| + | </ul> | ||
| + | <div class="image-container"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/8/85/T--Vilnius-Lithuania--Fig6_BAM_compl.png"/> | ||
| + | <p><strong>Fig. 6</strong> BamA after purification | ||
| + | 1 – BamA, 2 – BamA-HisN6, L – PageRuler Unstained Broad Range Protein Ladder</p> | ||
| + | </div> | ||
| + | <ul> | ||
| + | <Li>Bam B-D lipoproteins were expressed with the pelB signal sequence, leading them to be exported to the periplasm where lipidation takes place. We then isolated the proteins from the membrane fraction, which we solubilised with specific detergents before purification using Ni-NTA (Fig.7 and Fig.9) and gelfiltration (size-exclusion) (Fig. 8 and Fig. 9) columns. BamCDE were purified as a single subcomplex via one octahistidine tag on BamE.</Li> | ||
| + | </ul> | ||
| + | <h3>After purification with Ni-NTA column:</h3> | ||
| + | <p> | ||
| + | <div class="image-container"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/2/2c/T--Vilnius-Lithuania--Fig7_BAM_compl.png"/> | ||
| + | </p> | ||
| + | <p><strong>Fig. 7</strong> BamB after Ni-NTA column purification Lane L – PageRuler Unstained Protein Ladder, Lane 1 – Sample loaded on Ni-NTA Column, | ||
| + | Lanes 2-3 – Flow through fractions, Lanes 4-5 – washing fractions, Lanes 6-9 – Elution fractions | ||
| + | </p> | ||
| + | </div> | ||
| + | <h3> After gelfiltration:</h3> | ||
| + | |||
| + | <div class="image-container"> | ||
| + | <p> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/2/2e/T--Vilnius-Lithuania--Fig8_BAM_compl.png"/> | ||
| + | </p> | ||
| + | <p><strong>Fig. 8</strong> BamB fractions after gelfiltration | ||
| + | Lane L – PageRuler Unstained Protein Ladder, Lanes 1-14 Elution fractions | ||
| + | </p> | ||
| + | </div> | ||
| + | <h3> After gelfiltration:</h3> | ||
| + | |||
| + | <div class="image-container"> | ||
| + | |||
| + | <p> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/3/36/T--Vilnius-Lithuania--Fig9_BAM_compl.png"/> | ||
| + | </p> | ||
| + | <p><strong>Fig. 9</strong> BamCDE after Ni-NTA column purification Lane L – PageRuler Unstained Protein Ladder, Lane 1,2 - Flow through fractions, Lanes 3-4 – washing fractions, Lanes 5-7 – Elution fractions | ||
| + | </p> | ||
| + | <div> | ||
| + | <h3>After gelfiltration:</h3> | ||
| + | |||
| + | <div class="image-container"> | ||
| + | |||
| + | <p> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/a/ae/T--Vilnius-Lithuania--Fig10_BAM_compl.png"/> | ||
| + | </p> | ||
| + | <p><strong>Fig. 10</strong> BamCDE fractions after gelfiltration | ||
| + | Lane 1 – PageRuler Unstained Protein Ladder, 2-15 elution fractions</p> | ||
| + | </div> | ||
| + | |||
| + | <ul> | ||
| + | <li> | ||
| + | While SurA is a periplasmic protein, we had no issues overexpressing it in the cytoplasm for increased yield. We used a hexahistidine tag and purified using Ni-NTA (Fig.11) and gelfiltration (Fig.12) columns. | ||
| + | </li> | ||
| + | </ul> | ||
| + | <h3>After Ni-NTA column:</h3> | ||
| + | <div class="image-container"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/3/33/T--Vilnius-Lithuania--Fig11_BAM_compl.png"/> | ||
| + | <p><strong>Fig. 11 </strong>SurA after purification with Ni-NTA column | ||
| + | L - PageRuler Unstained Protein Ladder, 1 - Protein loaded on Ni-NTA Column, Lane 2 – Flow through fraction, 3 - washing fraction, 4-9 elution fractions | ||
| + | </p> | ||
| + | </div> | ||
| + | <h3>After gelfiltration</h3> | ||
| + | <div class="image-container"> | ||
| + | <p> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/e/e3/T--Vilnius-Lithuania--Fig12_BAM_compl.png"/> | ||
| + | </p> | ||
| + | <p><strong>Fig. 12 </strong>SurA fractions after gelfiltration | ||
| + | L - PageRuler Unstained Protein Ladder, 1-9 elution fractions | ||
| + | </p> | ||
| + | </div> | ||
| + | <H2>Folding assay</H2> | ||
| + | <p>To determine whether the purified proteins act as expected we conducted a specific a folding assay. Proteins possessing β-barrel structures exhibit a unique characteristic - when mixed with SDS (for SDS-PAGE) but unboiled, the β-barrel structure remains intact, which causes the protein to move differently in the SDS-PAGE gel in comparison to the same protein lacking these structures - which occurs when it is denatured or did not originally fold. Exploiting this characteristic makes it possible to observe and quantify protein folding levels.</p> | ||
| + | <p>For the first experiment we observed if BamB and BamCDE can incorporate the unfolded BamA protein into the membrane and reconstitute the complete BAM complex. This was accomplished by incubating SurA with BamA denatured in urea, then transferring it into a solution featuring liposomes, BamB and BamCDE, then further incubating for 2 hours. As BamA was expressed with a his-tag, we performed a blot to determine the level of protein folding (Fig. 13). </p> | ||
| + | <div class="image-container"> | ||
| + | <p> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/7/73/T--Vilnius-Lithuania--Fig13_BAM_compl.png"/> | ||
| + | </p> | ||
| + | <p><strong>Fig. 13</strong> Western blot of BamA folding. 1U - sample 1 unboiled, 1B - sample 1 boiled, 2U - sample 2 unboiled, 2B - sample 2 boiled, L - ladder | ||
| + | </p> | ||
| + | </div> | ||
| + | <p>As we can see from the results, after only 2 hours of incubation over 50% of BamA was processed and correctly folded, which is an indicator of proper functionality. At higher concentrations or within more enclosed environments, such as encapsulated within the liposome, efficiency is bound to increase.</p> | ||
| + | |||
| + | <h1>Conclusions</h1> | ||
| + | <p></p> | ||
| + | <p>We managed to successfully isolate the BAM protein complex at a high purity with relatively few steps. The BAM complex not only shows activity in vitro, it shows efficient activity within the presence of liposomes, which shows that it is functional and suitable for our system. | ||
| + | </p> | ||
| + | <p></p> | ||
| + | <h1>Discussion</h1> | ||
| + | |||
| + | |||
| + | <p></p> | ||
| + | <p>The study of MPs is and continues to be a difficult area of study, due to the sheer difficulty in handling them. As we have emphasized before, the integration of MP’s is a particularly pronounced issue, being borderline impossible for some cases in vitro. However, we have managed to demonstrate, that with our newly designed approach utilizing the BAM complex, the SynDrop system can allow for much more efficient insertion of MP’s as well as greatly expanding the total amount of viable MP’s for high throughput in vitro studies.</p> | ||
| + | <h2>References</h2> | ||
| + | <p> | ||
| + | <ol> | ||
| + | <Li>White, S. & Wimley, W. MEMBRANE PROTEIN FOLDING AND STABILITY: Physical Principles. Annual Review of Biophysics and Biomolecular Structure 28, 319-365 (1999).</Li> | ||
| + | <li>Noinaj, N., Rollauer, S. & Buchanan, S. The β-barrel membrane protein insertase machinery from Gram-negative bacteria. Current Opinion in Structural Biology 31, 35-42 (2015). | ||
| + | </li> | ||
| + | <li>Fleming, P. et al. BamA POTRA Domain Interacts with a Native Lipid Membrane Surface. Biophysical Journal 110, 2698-2709 (2016). | ||
| + | </li> | ||
| + | <li>Hussain, S. & Bernstein, H. The Bam complex catalyzes efficient insertion of bacterial outer membrane proteins into membrane vesicles of variable lipid composition. Journal of Biological Chemistry 293, 2959-2973 (2018). | ||
| + | </li> | ||
| + | <li>Driessen, A. & Nouwen, N. Protein Translocation Across the Bacterial Cytoplasmic Membrane. Annual Review of Biochemistry 77, 643-667 (2008). | ||
| + | </li> | ||
| + | <li>Sklar, J., Wu, T., Kahne, D. & Silhavy, T. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes & Development 21, 2473-2484 (2007). | ||
| + | </li> | ||
| + | <li> Bennion, D., Charlson, E., Coon, E. & Misra, R. Dissection of β-barrel outer membrane protein assembly pathways through characterizing BamA POTRA 1 mutants of Escherichia coli. Molecular Microbiology 77, 1153-1171 (2010). | ||
| + | </li> | ||
| + | <li>Gu, Y. et al. Structural basis of outer membrane protein insertion by the BAM complex. Nature 531, 64-69 (2016). | ||
| + | </li> | ||
| + | <li>Hagan, C., Westwood, D. & Kahne, D. Bam Lipoproteins Assemble BamA in Vitro. Biochemistry 52, 6108-6113 (2013). | ||
| + | </li> | ||
| + | |||
| + | </ol> | ||
| + | </p>- | ||
</div> | </div> | ||
</section> | </section> | ||
| Line 418: | Line 548: | ||
</p> | </p> | ||
<p> | <p> | ||
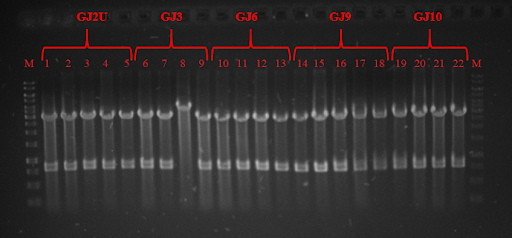
| − | pRSET plasmid and Sw<sub>x</sub> PCR products were digested with restriction enzymes and ligated, while GJ<sub>x</sub> PCR products were phosphorylated and ligated to produce plasmids from linear products. DH5α competent cells were transformed and plated on lysogeny broth (LB) media with ampicillin (Amp) and grown for 16 hours. Positive colonies were selected by colony PCR or restriction analysis (Fig. 3 and Fig. 4) and grown in 5 mL LB media. Plasmids were purified and BL21 competent cells were transformed. Three tubes of every construct plus plasmid with GFP without RNA thermometer were grown till OD<sub>600</sub> reached 0.4. Control samples were taken and protein expression was induced with Isopropyl β-D-1-thiogalactopyranoside (IPTG). One tube of every construct was grown in 24 ˚C, 30 ˚C, and 37 ˚C. Samples were taken after 1 and 2 hours. SDS-PAGE was run (for elaborate protocol see Notebook/<a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols">Protocols</a>). Fig. 5, Fig 6 and Fig. 7 depicts GFP expression at different temperatures. Although our RNA thermometers were designed to melt at 37 ˚C, some displayed leakiness to different extent. GJ3 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622011">BBa_K2622011</a>) RNA thermometer was the leakeast and allowed for GFP translation at lower temperatures. On the other hand, when grown at 37 ˚C, it unlocked the translation of GFP to highest yields. GJ2 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622010">BBa_K2622010</a>) was less leaky, but inhibited protein translation more strictly when grown at 37 ˚C. GJ6 (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622012">BBa_K2622012</a>), GJ9 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622013">BBa_K2622013</a>), and GJ10 (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622014">BBa_K2622014</a>) suppressed GFP production at 24 ˚C and 30 ˚C at similar level. They also inhibited translation to some extent at higher temperatures, meaning their melting temperature was not reached. Altogether these results prove, that our synthetic thermoswitches are temperature-responsive and act in physiological temperature range needed for IVTT reaction and also for BamA folding and membrane insertion. | + | pRSET plasmid and Sw<sub>x</sub> PCR products were digested with restriction enzymes and ligated, while GJ<sub>x</sub> PCR products were phosphorylated and ligated to produce plasmids from linear products. DH5α competent cells were transformed and plated on lysogeny broth (LB) media with ampicillin (Amp) and grown for 16 hours. Positive colonies were selected by colony PCR or restriction analysis (Fig. 3 and Fig. 4) and grown in 5 mL LB media. Plasmids were purified and BL21 competent cells were transformed. Three tubes of every construct plus plasmid with GFP without RNA thermometer were grown till OD<sub>600</sub> reached 0.4. Control samples were taken and protein expression was induced with Isopropyl β-D-1-thiogalactopyranoside (IPTG). One tube of every construct was grown in 24 ˚C, 30 ˚C, and 37 ˚C. Samples were taken after 1 and 2 hours. SDS-PAGE was run (for elaborate protocol see <a href="https://2018.igem.org/Team:Vilnius-Lithuania/LabBook">Notebook</a>/<a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols">Protocols</a>). Fig. 5, Fig. 6 and Fig. 7 depicts GFP expression at different temperatures. Although our RNA thermometers were designed to melt at 37 ˚C, some displayed leakiness to different extent. GJ3 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622011">BBa_K2622011</a>) RNA thermometer was the leakeast and allowed for GFP translation at lower temperatures. On the other hand, when grown at 37 ˚C, it unlocked the translation of GFP to highest yields. GJ2 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622010">BBa_K2622010</a>) was less leaky, but inhibited protein translation more strictly when grown at 37 ˚C. GJ6 (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622012">BBa_K2622012</a>), GJ9 (link:<a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622013">BBa_K2622013</a>), and GJ10 (link: <a href="http://parts.igem.org/wiki/index.php?title=Part:BBa_K2622014">BBa_K2622014</a>) suppressed GFP production at 24 ˚C and 30 ˚C at similar level. They also inhibited translation to some extent at higher temperatures, meaning their melting temperature was not reached. Altogether these results prove, that our synthetic thermoswitches are temperature-responsive and act in physiological temperature range needed for IVTT reaction and also for BamA folding and membrane insertion. |
</p> | </p> | ||
<p> | <p> | ||
| Line 519: | Line 649: | ||
</div> | </div> | ||
| − | Also, we checked if we could fuse MstX with other integral membrane proteins. In this case, IgA protein (Fig. 3). | + | <p>Also, we checked if we could fuse MstX with other integral membrane proteins. In this case, IgA protein (Fig. 3). |
</p> | </p> | ||
<p> | <p> | ||
| Line 576: | Line 706: | ||
<p>scFv consists of a minimal functional antigen-binding domain of an antibody (~30 kDa) (Fig. 1) , in which the heavy variable chain (VH) and light variable chain (VL) are connected by Ser and Gly rich flexible linker. [1] In most cases scFv is expressed in bacteria, where it is produced in cytoplasm, a reducing environment, in which disulfide bonds are not able to form and protein is quickly degraded or aggregated. Although poor solubility and affinity limit scFvs’ applications, their stability can be improved by merging with other proteins. [2] When expressed in cell free system, scFv should form disulfide bonds with the help of additional molecules. Merging to a membrane protein would provide additional stability and would display scFv on liposome membrane, where its activity could be detected. These improved qualities make ScFv recombinant proteins a perfect tool to evaluate, if SynDrop system acts in an anticipated manner. Of all possible scFvs we decided to use scFv-anti vaginolysin, which binds and neutralizes toxin vaginolysin (VLY). Its main advantage is rapid (< 1 h) and cheap detection of activity by inhibition of erythrocyte lysis (Fig. 2). Looking into future applications, scFvs are also attractive targets of molecular evolution, because one round of evolution would last less than one day thus generating a and wide range of different scFv mutants. Those displaying the highest affinity for antigens could be selected and used as drugs or drug carriers. </p> | <p>scFv consists of a minimal functional antigen-binding domain of an antibody (~30 kDa) (Fig. 1) , in which the heavy variable chain (VH) and light variable chain (VL) are connected by Ser and Gly rich flexible linker. [1] In most cases scFv is expressed in bacteria, where it is produced in cytoplasm, a reducing environment, in which disulfide bonds are not able to form and protein is quickly degraded or aggregated. Although poor solubility and affinity limit scFvs’ applications, their stability can be improved by merging with other proteins. [2] When expressed in cell free system, scFv should form disulfide bonds with the help of additional molecules. Merging to a membrane protein would provide additional stability and would display scFv on liposome membrane, where its activity could be detected. These improved qualities make ScFv recombinant proteins a perfect tool to evaluate, if SynDrop system acts in an anticipated manner. Of all possible scFvs we decided to use scFv-anti vaginolysin, which binds and neutralizes toxin vaginolysin (VLY). Its main advantage is rapid (< 1 h) and cheap detection of activity by inhibition of erythrocyte lysis (Fig. 2). Looking into future applications, scFvs are also attractive targets of molecular evolution, because one round of evolution would last less than one day thus generating a and wide range of different scFv mutants. Those displaying the highest affinity for antigens could be selected and used as drugs or drug carriers. </p> | ||
<div class="image-container"> | <div class="image-container"> | ||
| − | <img src="https://static.igem.org/mediawiki/2018/1/16/T--Vilnius-Lithuania--PERMATOMAS_ScFv.png" | + | <img src="https://static.igem.org/mediawiki/2018/1/16/T--Vilnius-Lithuania--PERMATOMAS_ScFv.png"/> |
| − | <p><strong> Fig. 1 </strong>Simplified structure of scFv Antibody</p> | + | <p><strong>Fig. 1 </strong>Simplified structure of scFv Antibody</p> |
</div> | </div> | ||
| − | |||
<div class="image-container"> | <div class="image-container"> | ||
| − | <img src="https://static.igem.org/mediawiki/2018/9/97/T--Vilnius-Lithuania--Fig2_NEW_real_Surface_scFV.png" | + | <img src="https://static.igem.org/mediawiki/2018/9/97/T--Vilnius-Lithuania--Fig2_NEW_real_Surface_scFV.png"/> |
| − | <p><strong> Fig. 2 </strong>Scheme of scFv_antiVLY and VLY interaction. Left- scFv_antiVLY binds to VLY, erythrocytes stay intact, Right- scFv_antiVLY does not bind and VLY lyse erythrocytes.</p> | + | <p><strong>Fig. 2 </strong>Scheme of scFv_antiVLY and VLY interaction. Left- scFv_antiVLY binds to VLY, erythrocytes stay intact, Right- scFv_antiVLY does not bind and VLY lyse erythrocytes.</p> |
| − | </div> | + | </div></p> |
<p></p> | <p></p> | ||
<h1>Results</h1> | <h1>Results</h1> | ||
| Line 589: | Line 718: | ||
<div class="image-container"></div> | <div class="image-container"></div> | ||
<p>scFv constructs were created <a href="http://parts.igem.org/Part:BBa_K2622004"> BBa_K2622004</a>. and checked by <a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols"> colony PCR and DNA sequencing</a>. scFv synthesis was performed in a cell free system. Validation of protein expression was done by running a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), see (Fig. 3)</p> | <p>scFv constructs were created <a href="http://parts.igem.org/Part:BBa_K2622004"> BBa_K2622004</a>. and checked by <a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols"> colony PCR and DNA sequencing</a>. scFv synthesis was performed in a cell free system. Validation of protein expression was done by running a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), see (Fig. 3)</p> | ||
| − | <img src="https://static.igem.org/mediawiki/2018/8/83/T--Vilnius-Lithuania--_Fig2_Surface-scFv.png" | + | <img src="https://static.igem.org/mediawiki/2018/8/83/T--Vilnius-Lithuania--_Fig2_Surface-scFv.png"> |
| − | + | ||
| − | + | <p><strong>Fig. 3 </strong> SDS-PAGE of scFv. GFP is used as positive control, C- chaperone DnaK.</p> | |
| − | <p><strong> Fig. 3 </strong> SDS-PAGE of scFv. GFP is used as positive control, C- chaperone DnaK.</p> | + | |
</div> | </div> | ||
<p>Red arrows in the photo indicate scFv anti-vaginolysin (~27 kDa). As successful synthesis was confirmed, the next step was to check if protein folded correctly and was able to bind its antigen - vaginolysin. We examined this by erythrocyte-lysis test, which was performed by comparing erythrocytes incubated with VLY (erythrocytes burst open) and erythrocytes incubated with VLY that was previously incubated with scFv anti-vaginolysin (less or no erythrocyte lysis). Results revealed that scFv binded to vaginolysin and inhibited cell lysis. Graph in (Fig. 4) demonstrates that scFv indeed attenuated the lysis of erythrocytes. These result prove scFv activity in IVTT system.</p> | <p>Red arrows in the photo indicate scFv anti-vaginolysin (~27 kDa). As successful synthesis was confirmed, the next step was to check if protein folded correctly and was able to bind its antigen - vaginolysin. We examined this by erythrocyte-lysis test, which was performed by comparing erythrocytes incubated with VLY (erythrocytes burst open) and erythrocytes incubated with VLY that was previously incubated with scFv anti-vaginolysin (less or no erythrocyte lysis). Results revealed that scFv binded to vaginolysin and inhibited cell lysis. Graph in (Fig. 4) demonstrates that scFv indeed attenuated the lysis of erythrocytes. These result prove scFv activity in IVTT system.</p> | ||
<div class="image-container"> | <div class="image-container"> | ||
| − | <img src="https://static.igem.org/mediawiki/2018/7/7b/T--Vilnius-Lithuania--_Fig3_Surface-scFv.png" | + | <img src="https://static.igem.org/mediawiki/2018/7/7b/T--Vilnius-Lithuania--_Fig3_Surface-scFv.png"> |
| − | + | ||
<p> | <p> | ||
| − | <p><strong> Fig. 4 </strong> Percentage of erythrocyte lysis at different +/-scFv dilutions.</p> | + | <p><strong>Fig. 4 </strong> Percentage of erythrocyte lysis at different +/-scFv dilutions.</p> |
</div> | </div> | ||
<p>We then went one step further and constructed MstX-scFv_antiVLY <a href="http://parts.igem.org/Part:BBa_K2622038"> BBa_2622038</a>, fusion protein, aiming to increase the stability of scFv having in mind future applications and experiments of exposing it on liposome surface. Fusion protein was expressed in E.coli cells; yellow to red arrows in (Fig. 5A) indicate MstX-scFv expression after induction with IPTG.</p> | <p>We then went one step further and constructed MstX-scFv_antiVLY <a href="http://parts.igem.org/Part:BBa_K2622038"> BBa_2622038</a>, fusion protein, aiming to increase the stability of scFv having in mind future applications and experiments of exposing it on liposome surface. Fusion protein was expressed in E.coli cells; yellow to red arrows in (Fig. 5A) indicate MstX-scFv expression after induction with IPTG.</p> | ||
<p>Finally, we expressed the protein in a cell free system (Fig. 5B) along with scFv in order to compare how well scFv accomplishes its function alone or binded to other protein. In this case MstX-scFv_antiVLY fusion did not show superior activity than scFv_antiVLY alone (Fig. 6). These results also reveal that scFv_antiVLY is very sensitive and loses its activity with time. Ist and IInd attempts were separated by 1-2 hours. This amount of time is enough to measure decreasing activity. This must be taken into account when performing future experiments.</p> | <p>Finally, we expressed the protein in a cell free system (Fig. 5B) along with scFv in order to compare how well scFv accomplishes its function alone or binded to other protein. In this case MstX-scFv_antiVLY fusion did not show superior activity than scFv_antiVLY alone (Fig. 6). These results also reveal that scFv_antiVLY is very sensitive and loses its activity with time. Ist and IInd attempts were separated by 1-2 hours. This amount of time is enough to measure decreasing activity. This must be taken into account when performing future experiments.</p> | ||
| − | <img src="https://static.igem.org/mediawiki/2018/9/9c/T--Vilnius-Lithuania--Fig_4._5._Surface_scFv.png" | + | <img src="https://static.igem.org/mediawiki/2018/9/9c/T--Vilnius-Lithuania--Fig_4._5._Surface_scFv.png"> |
| − | + | ||
<p> | <p> | ||
| − | <p><strong> Fig. 5 </strong>A- MstX-scFv_antiVLY expression in Escherichia coli. B- scFv_antiVLY and MstX-scFv_antiVLY expression in cell-free system.</p> | + | <p><strong>Fig. 5 </strong>A- MstX-scFv_antiVLY expression in Escherichia coli. B- scFv_antiVLY and MstX-scFv_antiVLY expression in cell-free system.</p> |
| − | <img src="https://static.igem.org/mediawiki/2018/0/0c/T--Vilnius-Lithuania--_Fig6_Surface-scFv.png" | + | <img src="https://static.igem.org/mediawiki/2018/0/0c/T--Vilnius-Lithuania--_Fig6_Surface-scFv.png"> |
| − | + | ||
| − | + | ||
| − | + | ||
<p> | <p> | ||
| − | <p><strong>Fig 6 </strong> Percentage of erythrocyte lysis at different scFv/MstX-scFv dilutions.</p> | + | <p><strong>Fig. 6 </strong> Percentage of erythrocyte lysis at different scFv/MstX-scFv dilutions.</p> |
<h1>Conclusions</h1> | <h1>Conclusions</h1> | ||
<p> | <p> | ||
| Line 634: | Line 757: | ||
</section> | </section> | ||
</div> | </div> | ||
| − | |||
</div> | </div> | ||
<div class="carrot-back"> | <div class="carrot-back"> | ||
Latest revision as of 20:11, 30 November 2018
Design and Results
Results
Cell-free, synthetic biology systems open new horizons in engineering biomolecular systems which feature complex, cell-like behaviors in the absence of living entities. Having no superior genetic control, user-controllable mechanisms to regulate gene expression are necessary to successfully operate these systems. We have created a small collection of synthetic RNA thermometers that enable temperature-dependent translation of membrane proteins, work well in cells and display great potential to be transferred to any in vitro protein synthesis system.