Justas2010 (Talk | contribs) |
Kasparas12 (Talk | contribs) |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 61: | Line 61: | ||
<img src="https://static.igem.org/mediawiki/2018/0/0e/T--Vilnius-Lithuania--Fig2_Liposomes.png"/> | <img src="https://static.igem.org/mediawiki/2018/0/0e/T--Vilnius-Lithuania--Fig2_Liposomes.png"/> | ||
<p><strong>Fig. 2 a </strong>AutoCAD design for the photomask. There are 16 individual microchannel devices on a | <p><strong>Fig. 2 a </strong>AutoCAD design for the photomask. There are 16 individual microchannel devices on a | ||
| − | + | ||
| + | single chip. <strong>b</strong> One device consists of three inlets, an outlet and a star-shaped junction.</p> | ||
</p> | </p> | ||
</div> | </div> | ||
| Line 349: | Line 350: | ||
<img src="https://static.igem.org/mediawiki/2018/1/1c/T--Vilnius-Lithuania--Fig1_BAM_compl.png"/> | <img src="https://static.igem.org/mediawiki/2018/1/1c/T--Vilnius-Lithuania--Fig1_BAM_compl.png"/> | ||
<p><strong>Fig. 1</strong> Structure of BAM complex</p> | <p><strong>Fig. 1</strong> Structure of BAM complex</p> | ||
| − | + | </div> | |
<h2>Outer membrane protein (OMP) insertion into membrane in bacteria cells | <h2>Outer membrane protein (OMP) insertion into membrane in bacteria cells | ||
</h2> | </h2> | ||
| Line 355: | Line 356: | ||
<img src="https://static.igem.org/mediawiki/2018/3/3a/T--Vilnius-Lithuania--Fig2_BAM_compl.png"/> | <img src="https://static.igem.org/mediawiki/2018/3/3a/T--Vilnius-Lithuania--Fig2_BAM_compl.png"/> | ||
<p><strong>Fig. 2</strong> 5 steps scheme of OMP insertion into OM.</p> | <p><strong>Fig. 2</strong> 5 steps scheme of OMP insertion into OM.</p> | ||
| + | </div> | ||
<p>Generally MPs are integrated during translation with the assistance of the Sec Translocon. However, as there is no protein translation within the periplasm, OMPs require an alternative integration mechanism. | <p>Generally MPs are integrated during translation with the assistance of the Sec Translocon. However, as there is no protein translation within the periplasm, OMPs require an alternative integration mechanism. | ||
| Line 368: | Line 370: | ||
<img src="https://static.igem.org/mediawiki/2018/3/30/T--Vilnius-Lithuania--Fig3_BAM_compl.png"/> | <img src="https://static.igem.org/mediawiki/2018/3/30/T--Vilnius-Lithuania--Fig3_BAM_compl.png"/> | ||
<p><strong>Fig. 3</strong> Bam lipoproteins assemble BamA in vitro</p> | <p><strong>Fig. 3</strong> Bam lipoproteins assemble BamA in vitro</p> | ||
| + | </div> | ||
<p></p> | <p></p> | ||
<h1>Results</h1> | <h1>Results</h1> | ||
| Line 384: | Line 387: | ||
</p> | </p> | ||
<div class="image-container"> | <div class="image-container"> | ||
| − | <img src="https://static.igem.org/mediawiki/2018/5/54/T--Vilnius-Lithuania--Fig4.1_BAM_compl.png"/ | + | <img src="https://static.igem.org/mediawiki/2018/5/54/T--Vilnius-Lithuania--Fig4.1_BAM_compl.png"/> |
| − | <img src="https://static.igem.org/mediawiki/2018/3/3d/T--Vilnius-Lithuania--Fig4.2_BAM_compl.png"/ | + | <img src="https://static.igem.org/mediawiki/2018/3/3d/T--Vilnius-Lithuania--Fig4.2_BAM_compl.png"/> |
<p><strong>Fig. 4</strong> Maps of constructed plasmids</p> | <p><strong>Fig. 4</strong> Maps of constructed plasmids</p> | ||
| − | <img src="https://static.igem.org/mediawiki/2018/2/2e/T--Vilnius-Lithuania--Fig5_BAM_compl.png"/ | + | <img src="https://static.igem.org/mediawiki/2018/2/2e/T--Vilnius-Lithuania--Fig5_BAM_compl.png"/> |
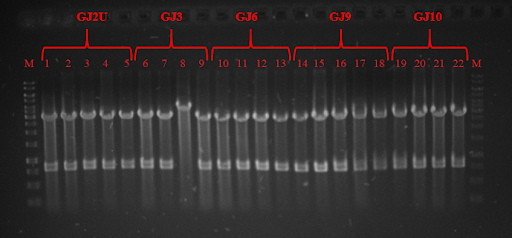
<p><strong>Fig. 5</strong> PCR products of genes of BAM complex 1,18 – GeneRuler 1 kb DNA ladder; 4,5 – SurA (1250bp); 6,7 - BamE (390bp); 8,9 – BamB (1201bp); 10, 11 – BamC (1056bp); 12, 13 – BamD(756bp); 14,16 – BamA (2455bp)</p> | <p><strong>Fig. 5</strong> PCR products of genes of BAM complex 1,18 – GeneRuler 1 kb DNA ladder; 4,5 – SurA (1250bp); 6,7 - BamE (390bp); 8,9 – BamB (1201bp); 10, 11 – BamC (1056bp); 12, 13 – BamD(756bp); 14,16 – BamA (2455bp)</p> | ||
| + | </div> | ||
<h2> | <h2> | ||
Protein purification | Protein purification | ||
| Line 400: | Line 404: | ||
<p><strong>Fig. 6</strong> BamA after purification | <p><strong>Fig. 6</strong> BamA after purification | ||
1 – BamA, 2 – BamA-HisN6, L – PageRuler Unstained Broad Range Protein Ladder</p> | 1 – BamA, 2 – BamA-HisN6, L – PageRuler Unstained Broad Range Protein Ladder</p> | ||
| + | </div> | ||
<ul> | <ul> | ||
<Li>Bam B-D lipoproteins were expressed with the pelB signal sequence, leading them to be exported to the periplasm where lipidation takes place. We then isolated the proteins from the membrane fraction, which we solubilised with specific detergents before purification using Ni-NTA (Fig.7 and Fig.9) and gelfiltration (size-exclusion) (Fig. 8 and Fig. 9) columns. BamCDE were purified as a single subcomplex via one octahistidine tag on BamE.</Li> | <Li>Bam B-D lipoproteins were expressed with the pelB signal sequence, leading them to be exported to the periplasm where lipidation takes place. We then isolated the proteins from the membrane fraction, which we solubilised with specific detergents before purification using Ni-NTA (Fig.7 and Fig.9) and gelfiltration (size-exclusion) (Fig. 8 and Fig. 9) columns. BamCDE were purified as a single subcomplex via one octahistidine tag on BamE.</Li> | ||
| Line 405: | Line 410: | ||
<h3>After purification with Ni-NTA column:</h3> | <h3>After purification with Ni-NTA column:</h3> | ||
<p> | <p> | ||
| + | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/2/2c/T--Vilnius-Lithuania--Fig7_BAM_compl.png"/> | <img src="https://static.igem.org/mediawiki/2018/2/2c/T--Vilnius-Lithuania--Fig7_BAM_compl.png"/> | ||
</p> | </p> | ||
| Line 410: | Line 416: | ||
Lanes 2-3 – Flow through fractions, Lanes 4-5 – washing fractions, Lanes 6-9 – Elution fractions | Lanes 2-3 – Flow through fractions, Lanes 4-5 – washing fractions, Lanes 6-9 – Elution fractions | ||
</p> | </p> | ||
| + | </div> | ||
<h3> After gelfiltration:</h3> | <h3> After gelfiltration:</h3> | ||
| + | <div class="image-container"> | ||
<p> | <p> | ||
<img src="https://static.igem.org/mediawiki/2018/2/2e/T--Vilnius-Lithuania--Fig8_BAM_compl.png"/> | <img src="https://static.igem.org/mediawiki/2018/2/2e/T--Vilnius-Lithuania--Fig8_BAM_compl.png"/> | ||
| Line 418: | Line 426: | ||
Lane L – PageRuler Unstained Protein Ladder, Lanes 1-14 Elution fractions | Lane L – PageRuler Unstained Protein Ladder, Lanes 1-14 Elution fractions | ||
</p> | </p> | ||
| + | </div> | ||
<h3> After gelfiltration:</h3> | <h3> After gelfiltration:</h3> | ||
| + | |||
| + | <div class="image-container"> | ||
<p> | <p> | ||
| Line 425: | Line 436: | ||
<p><strong>Fig. 9</strong> BamCDE after Ni-NTA column purification Lane L – PageRuler Unstained Protein Ladder, Lane 1,2 - Flow through fractions, Lanes 3-4 – washing fractions, Lanes 5-7 – Elution fractions | <p><strong>Fig. 9</strong> BamCDE after Ni-NTA column purification Lane L – PageRuler Unstained Protein Ladder, Lane 1,2 - Flow through fractions, Lanes 3-4 – washing fractions, Lanes 5-7 – Elution fractions | ||
</p> | </p> | ||
| + | <div> | ||
<h3>After gelfiltration:</h3> | <h3>After gelfiltration:</h3> | ||
| + | |||
| + | <div class="image-container"> | ||
<p> | <p> | ||
| Line 431: | Line 445: | ||
</p> | </p> | ||
<p><strong>Fig. 10</strong> BamCDE fractions after gelfiltration | <p><strong>Fig. 10</strong> BamCDE fractions after gelfiltration | ||
| − | Lane 1 – PageRuler Unstained Protein Ladder, 2-15 elution fractions</p><ul> | + | Lane 1 – PageRuler Unstained Protein Ladder, 2-15 elution fractions</p> |
| + | </div> | ||
| + | |||
| + | <ul> | ||
<li> | <li> | ||
While SurA is a periplasmic protein, we had no issues overexpressing it in the cytoplasm for increased yield. We used a hexahistidine tag and purified using Ni-NTA (Fig.11) and gelfiltration (Fig.12) columns. | While SurA is a periplasmic protein, we had no issues overexpressing it in the cytoplasm for increased yield. We used a hexahistidine tag and purified using Ni-NTA (Fig.11) and gelfiltration (Fig.12) columns. | ||
| Line 437: | Line 454: | ||
</ul> | </ul> | ||
<h3>After Ni-NTA column:</h3> | <h3>After Ni-NTA column:</h3> | ||
| + | <div class="image-container"> | ||
<img src="https://static.igem.org/mediawiki/2018/3/33/T--Vilnius-Lithuania--Fig11_BAM_compl.png"/> | <img src="https://static.igem.org/mediawiki/2018/3/33/T--Vilnius-Lithuania--Fig11_BAM_compl.png"/> | ||
<p><strong>Fig. 11 </strong>SurA after purification with Ni-NTA column | <p><strong>Fig. 11 </strong>SurA after purification with Ni-NTA column | ||
L - PageRuler Unstained Protein Ladder, 1 - Protein loaded on Ni-NTA Column, Lane 2 – Flow through fraction, 3 - washing fraction, 4-9 elution fractions | L - PageRuler Unstained Protein Ladder, 1 - Protein loaded on Ni-NTA Column, Lane 2 – Flow through fraction, 3 - washing fraction, 4-9 elution fractions | ||
</p> | </p> | ||
| + | </div> | ||
<h3>After gelfiltration</h3> | <h3>After gelfiltration</h3> | ||
| + | <div class="image-container"> | ||
<p> | <p> | ||
<img src="https://static.igem.org/mediawiki/2018/e/e3/T--Vilnius-Lithuania--Fig12_BAM_compl.png"/> | <img src="https://static.igem.org/mediawiki/2018/e/e3/T--Vilnius-Lithuania--Fig12_BAM_compl.png"/> | ||
| Line 447: | Line 467: | ||
<p><strong>Fig. 12 </strong>SurA fractions after gelfiltration | <p><strong>Fig. 12 </strong>SurA fractions after gelfiltration | ||
L - PageRuler Unstained Protein Ladder, 1-9 elution fractions | L - PageRuler Unstained Protein Ladder, 1-9 elution fractions | ||
| − | </p><H2>Folding assay</H2> | + | </p> |
| + | </div> | ||
| + | <H2>Folding assay</H2> | ||
<p>To determine whether the purified proteins act as expected we conducted a specific a folding assay. Proteins possessing β-barrel structures exhibit a unique characteristic - when mixed with SDS (for SDS-PAGE) but unboiled, the β-barrel structure remains intact, which causes the protein to move differently in the SDS-PAGE gel in comparison to the same protein lacking these structures - which occurs when it is denatured or did not originally fold. Exploiting this characteristic makes it possible to observe and quantify protein folding levels.</p> | <p>To determine whether the purified proteins act as expected we conducted a specific a folding assay. Proteins possessing β-barrel structures exhibit a unique characteristic - when mixed with SDS (for SDS-PAGE) but unboiled, the β-barrel structure remains intact, which causes the protein to move differently in the SDS-PAGE gel in comparison to the same protein lacking these structures - which occurs when it is denatured or did not originally fold. Exploiting this characteristic makes it possible to observe and quantify protein folding levels.</p> | ||
<p>For the first experiment we observed if BamB and BamCDE can incorporate the unfolded BamA protein into the membrane and reconstitute the complete BAM complex. This was accomplished by incubating SurA with BamA denatured in urea, then transferring it into a solution featuring liposomes, BamB and BamCDE, then further incubating for 2 hours. As BamA was expressed with a his-tag, we performed a blot to determine the level of protein folding (Fig. 13). </p> | <p>For the first experiment we observed if BamB and BamCDE can incorporate the unfolded BamA protein into the membrane and reconstitute the complete BAM complex. This was accomplished by incubating SurA with BamA denatured in urea, then transferring it into a solution featuring liposomes, BamB and BamCDE, then further incubating for 2 hours. As BamA was expressed with a his-tag, we performed a blot to determine the level of protein folding (Fig. 13). </p> | ||
| + | <div class="image-container"> | ||
<p> | <p> | ||
<img src="https://static.igem.org/mediawiki/2018/7/73/T--Vilnius-Lithuania--Fig13_BAM_compl.png"/> | <img src="https://static.igem.org/mediawiki/2018/7/73/T--Vilnius-Lithuania--Fig13_BAM_compl.png"/> | ||
| Line 455: | Line 478: | ||
<p><strong>Fig. 13</strong> Western blot of BamA folding. 1U - sample 1 unboiled, 1B - sample 1 boiled, 2U - sample 2 unboiled, 2B - sample 2 boiled, L - ladder | <p><strong>Fig. 13</strong> Western blot of BamA folding. 1U - sample 1 unboiled, 1B - sample 1 boiled, 2U - sample 2 unboiled, 2B - sample 2 boiled, L - ladder | ||
</p> | </p> | ||
| + | </div> | ||
<p>As we can see from the results, after only 2 hours of incubation over 50% of BamA was processed and correctly folded, which is an indicator of proper functionality. At higher concentrations or within more enclosed environments, such as encapsulated within the liposome, efficiency is bound to increase.</p> | <p>As we can see from the results, after only 2 hours of incubation over 50% of BamA was processed and correctly folded, which is an indicator of proper functionality. At higher concentrations or within more enclosed environments, such as encapsulated within the liposome, efficiency is bound to increase.</p> | ||
Latest revision as of 20:11, 30 November 2018
Design and Results
Results
Cell-free, synthetic biology systems open new horizons in engineering biomolecular systems which feature complex, cell-like behaviors in the absence of living entities. Having no superior genetic control, user-controllable mechanisms to regulate gene expression are necessary to successfully operate these systems. We have created a small collection of synthetic RNA thermometers that enable temperature-dependent translation of membrane proteins, work well in cells and display great potential to be transferred to any in vitro protein synthesis system.