Elinramstrom (Talk | contribs) |
MatildaBrink (Talk | contribs) |
||
| (43 intermediate revisions by 8 users not shown) | |||
| Line 5: | Line 5: | ||
{{Uppsala/javascript/scroll-button}} | {{Uppsala/javascript/scroll-button}} | ||
{{Uppsala/javascript/redirect_js}} | {{Uppsala/javascript/redirect_js}} | ||
| + | {{Uppsala/buttons}} | ||
<html> | <html> | ||
<head> | <head> | ||
| − | <style type="text/css"> | + | <style type="text/css"> |
| + | |||
| + | .parallax { | ||
| + | /* The image used */ | ||
| + | background-image: url("https://static.igem.org/mediawiki/2018/f/f3/T--Uppsala--HEADER_INTERLAB_3.jpeg"); | ||
| + | } | ||
| − | + | ||
| − | + | ||
| + | |||
| + | |||
| + | </style> | ||
| + | |||
| + | |||
| + | </head> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <div class="svg-wrapper" id="Project_Description"> | ||
| + | |||
| + | |||
| + | |||
| + | <svg version="1.1" id="Layer_1" xmlns="http://www.w3.org/2000/svg" xmlns:xlink="http://www.w3.org/1999/xlink" x="0px" y="0px" | ||
| + | viewBox="0 0 2041 335" style="enable-background:new 0 0 2041 335;" xml:space="preserve"> | ||
| + | <style type="text/css"> | ||
| + | .st0{fill:#fff;stroke:#FFFFFF;stroke-width:3;stroke-miterlimit:10;} | ||
| + | </style> | ||
| + | <g> | ||
| + | <path class="st0" d="M22.8,31.3l24.4,0.8c16,0.5,26.5,0.8,31.4,0.8c4.4,0,23.1-0.5,56.1-1.6l1.6,1.4v9.8l-1.6,1.4 | ||
| + | c-9,0.3-15.9,0.7-20.8,1.2c-4.9,0.5-8.4,1.5-10.6,2.9c-2.2,1.4-3.7,4.8-4.4,10.1c-0.7,5.3-1.1,12.6-1.1,22l-0.4,44.9v90.4l0.2,38.1 | ||
| + | c0.1,14.7,0.5,24.2,1.2,28.5c0.6,4.3,1.6,7.2,2.8,8.6c1.2,1.4,3.6,2.6,7.1,3.6s12.2,1.7,26,2.2l1.6,1.2v9.8l-1.6,1.6 | ||
| + | c-0.7,0-12.1-0.4-34.4-1.2c-9.2-0.3-16.5-0.4-21.7-0.4c-4,0-22.7,0.5-55.9,1.6l-1.6-1.6v-9.8l1.6-1.2c12.1-0.5,20.2-1.2,24.2-2 | ||
| + | c4-0.8,6.7-1.8,8.1-3c1.4-1.2,2.5-3.9,3.3-7.9c0.8-4,1.3-13,1.5-27l0.2-41.2v-90.4l-0.4-38.1c-0.1-14.8-0.5-24.4-1.1-28.7 | ||
| + | s-1.5-7.2-2.7-8.6c-1.2-1.4-3.6-2.6-7.1-3.5c-3.5-0.9-12.2-1.6-26-2.1l-1.6-1.4v-9.8L22.8,31.3z"/> | ||
| + | <path class="st0" d="M158.9,308.6l-1.8-1.8v-9l1.8-1.6c7.9-0.1,14.3-0.6,19-1.4c4.8-0.8,7.8-2.1,9.2-3.9c1.4-1.8,2.3-4.8,2.7-8.8 | ||
| + | c0.5-4,0.9-13.1,1.4-27.3c0.5-14.2,0.7-24.3,0.7-30.5V66.6c0-6.6-1.2-11.6-3.6-14.7c-2.4-3.2-5.7-5.2-9.9-6.1 | ||
| + | c-4.2-0.8-11.3-1.3-21.3-1.5l-1.6-1.6v-9.2l1.6-1.8c18,0.7,29.8,1,35.4,1c13.1,0,24-0.3,32.4-1l62.3,80.9l91.6,114.8 | ||
| + | c11.1,13.9,22.3,27.1,33.8,39.6V116.1c0-4.7-0.2-14.7-0.7-30c-0.5-15.3-1-25-1.7-29.1c-0.7-4.1-1.8-6.9-3.4-8.3 | ||
| + | c-1.6-1.4-4.7-2.5-9.2-3.2c-4.5-0.7-10.6-1.1-18.3-1.3l-1.6-1.6v-9l1.6-1.8c10.5,0.7,25.6,1,45.1,1c17.8,0,30.8-0.3,38.9-1l1.8,1.8 | ||
| + | v9l-1.6,1.6c-8.6,0.1-15.2,0.6-19.9,1.5c-4.7,0.8-7.6,2.2-8.8,4.2s-2,4.8-2.4,8.6c-0.5,3.8-0.9,12.8-1.4,27 | ||
| + | c-0.5,14.2-0.7,24.4-0.7,30.7v92.8l0.2,53.7c0.3,17.6,0.5,35.2,0.8,52.9c-6.5-2.2-17.8-5-33.8-8.2l-13.9-16.4l-41.6-50.8 | ||
| + | L209.7,74.7v149.8c0,6,0.2,16.7,0.7,32c0.5,15.4,1,24.7,1.7,27.9c0.6,3.3,1.5,5.5,2.5,6.7c1,1.2,3.3,2.4,6.7,3.4 | ||
| + | c3.4,1,10.6,1.6,21.4,1.8l1.6,1.6v9.2l-1.6,1.6c-6.8-0.9-17.7-1.4-32.8-1.4C190.5,307.3,173.5,307.7,158.9,308.6z"/> | ||
| + | <path class="st0" d="M714.2,31.3l1.8,1.8c-1.2,11.7-1.8,32.2-1.8,61.3l-1.6,1.6h-9.2l-1.6-1.8l-2.1-18c-1-9.6-1.9-15.5-2.4-17.7 | ||
| + | s-1.5-3.7-2.8-4.7c-1.3-1-5.5-1.8-12.7-2.4c-7.2-0.6-15.2-1-24-1h-36.9c-1,29.4-1.6,46.4-1.6,51v113.9l0.4,38.1 | ||
| + | c0.1,14.7,0.5,24.3,1.1,28.6c0.6,4.4,1.5,7.2,2.7,8.6c1.2,1.4,3.6,2.5,7.1,3.5s12.2,1.7,26.2,2.2l1.4,1.2v9.8l-1.4,1.6 | ||
| + | c-33.3-1-52-1.6-55.9-1.6c-4.2,0-22.7,0.5-55.7,1.6l-1.6-1.6v-9.8l1.6-1.2c12.2-0.5,20.3-1.2,24.3-2c4-0.8,6.6-1.8,8-3 | ||
| + | c1.4-1.2,2.5-3.9,3.4-7.9c0.9-4,1.4-13,1.4-27l0.4-41.2V101.4c0-2.1-0.5-19.1-1.6-51h-29.1c-12,0-22.3,0.3-31.1,0.8 | ||
| + | c-8.7,0.5-13.8,1.9-15.1,4s-2.6,9.1-3.8,21l-2,18l-1.8,1.8h-9l-1.6-1.6c-0.3-31.4-0.8-51.8-1.8-61.3l1.8-1.8 | ||
| + | c22.7,1,60.4,1.6,113.1,1.6C654.7,32.9,692.5,32.3,714.2,31.3z"/> | ||
| + | <path class="st0" d="M737.8,32.7l1.6-1.4c44.7,0.8,73.2,1.2,85.7,1.2c42.7,0,80.7-0.4,114.1-1.2l1,1.4c-3.3,12.5-5.7,32.1-7.2,58.8 | ||
| + | l-1.2,1.6h-9.6l-1.8-1.6c0.1-1.2,0.2-2.2,0.2-3.1c0-11.1-0.8-21.6-2.5-31.6c-8.2-3.6-19.6-6-34.1-7.1c-14.5-1.1-25.4-1.7-32.7-1.7 | ||
| + | c-10.3,0-23.4,0.7-39.5,2.1l-0.8,31.3l-0.6,35.7l-0.6,39.6c14.1,0.8,27,1.2,38.9,1.2c12,0,22-0.4,30.1-1.2 | ||
| + | c8.1-0.8,12.8-1.6,14.2-2.4c1.4-0.8,2.4-2.5,3.1-4.9c0.7-2.4,1.3-6.7,1.9-12.8l0.8-12.1l1.6-1.6h9l1.6,1.6 | ||
| + | c-0.3,14.2-0.6,28.5-1,42.8l1,43.9l-1.6,1.6h-9l-1.6-1.6l-1.4-11.7c-1.2-9.1-2-14.6-2.5-16.3c-0.5-1.8-1.7-3.3-3.6-4.6 | ||
| + | c-1.9-1.3-6.7-2.3-14.6-3.1s-18.6-1.2-32.2-1.2c-7.6,0-19.1,0.3-34.6,1c-0.7,10.9-1,29.2-1,54.7c0,27.3,0.3,47.5,1,60.4h35.9 | ||
| + | c31.6,0,51.4-0.4,59.4-1.2c7.9-0.8,15.5-2.7,22.7-5.7c1.2-3,3.1-9.9,5.8-20.8c2.7-10.9,4.2-17.7,4.6-20.4l2-1.4h9.4l1.6,1.4 | ||
| + | c-1.2,5.6-2.3,15.4-3.4,29.3c-1.1,13.9-1.7,25.8-1.7,35.7l-1.4,1.6c-24.9-0.8-57.4-1.2-97.7-1.2l-53.1-0.4 | ||
| + | c-12.4,0-25.3,0.5-38.7,1.6l-1.6-1.6v-6.4l1.6-2c7.7-3.9,12.1-6.3,13.2-7.3c1.1-1,2-12.4,2.7-34.2c0.7-21.8,1.1-38.4,1.1-49.9 | ||
| + | v-71.7L772,91.5c0-9.6-0.1-17.5-0.4-23.5c-0.3-6.1-0.7-10.4-1.4-13c-0.7-2.6-1.4-4.4-2.2-5.5c-0.8-1-2.2-1.9-4.1-2.6 | ||
| + | c-1.9-0.7-5.1-1.3-9.7-1.9l-14.8-1.2l-1.6-1.4V32.7z"/> | ||
| + | <path class="st0" d="M976.1,32.7l1.6-1.4c23.8,1,42.4,1.6,55.7,1.6c14.7,0,34.6-0.5,59.6-1.4c9.2-0.3,15.9-0.4,20.1-0.4 | ||
| + | c32.9,0,55.3,6,67,18c11.7,12,17.6,26.2,17.6,42.6c0,10.9-2.9,21.7-8.6,32.2c-5.7,10.5-14.2,19.6-25.4,27.1 | ||
| + | c-11.2,7.5-26.6,13.1-46.3,16.9c3.6,5.1,10.2,13.9,19.7,26.4l27.7,36.7c9,12.1,18.6,24.9,28.9,38.4c10.3,13.5,16.9,21.1,19.9,22.9 | ||
| + | c3,1.8,5.6,2.8,7.7,3.2s5.6,0.6,10.3,0.6l1.6,1.6v9.2l-1.8,1.8c-6.3-0.8-13.7-1.2-22.3-1.2c-9.2,0-19.3,0.4-30.3,1.2 | ||
| + | c-9-10.9-23.1-29.9-42.4-56.8c-22.4-31.3-45.3-60.2-68.8-86.9l2-6.1c6.8,0.4,12,0.6,15.8,0.6c22.5,0,40.2-5.4,52.9-16.2 | ||
| + | c12.8-10.8,19.1-25.8,19.1-44.9c0-17.2-5.7-30.2-17.2-39c-11.5-8.8-27-13.2-46.5-13.2c-13.7,0-26.6,1.9-38.7,5.7 | ||
| + | c-2,2.9-2.9,27.3-2.9,73.2v90.4l0.2,38.1c0.1,14.7,0.5,24.2,1.2,28.5c0.7,4.3,1.6,7.2,2.8,8.6c1.2,1.4,3.6,2.6,7.1,3.6 | ||
| + | s12.2,1.7,26,2.2l1.6,1.2v9.8l-1.6,1.6c-0.7,0-12.1-0.4-34.4-1.2c-9.2-0.3-16.5-0.4-21.7-0.4c-4,0-22.7,0.5-55.9,1.6l-1.6-1.6v-9.8 | ||
| + | l1.6-1.2c12.1-0.5,20.2-1.2,24.2-2c4-0.8,6.7-1.8,8.1-3c1.4-1.2,2.5-3.9,3.3-7.9c0.8-4,1.3-13,1.5-27l0.2-41.2v-90.4l-0.4-38.1 | ||
| + | c-0.1-14.8-0.5-24.4-1.1-28.7s-1.5-7.2-2.7-8.6c-1.2-1.4-3.6-2.6-7.1-3.5c-3.5-0.9-12.2-1.6-26-2.1l-1.6-1.4V32.7z"/> | ||
| + | <path class="st0" d="M1242.3,32.7l1.6-1.4c32.3,1,50.9,1.6,55.9,1.6c4.4,0,23.1-0.5,56.1-1.6l1.6,1.4v9.8l-1.6,1.4 | ||
| + | c-9,0.3-15.9,0.7-20.8,1.2c-4.9,0.5-8.4,1.5-10.6,2.9c-2.2,1.4-3.7,4.8-4.4,10.1c-0.7,5.3-1.1,12.6-1.1,22l-0.4,44.9v86.3 | ||
| + | c0,21.6,0.1,36.7,0.4,45.3l1,33.8h39.8c31.8,0,51.6-0.4,59.5-1.2c7.9-0.8,15.5-2.7,22.8-5.7c1.4-3.3,3.5-10.7,6.2-22.5 | ||
| + | c2.7-11.7,4.1-18.5,4.2-20.3l1.8-1.6h9.6l1.4,1.6c-1,5.2-2.1,15.3-3.2,30.3c-1.1,15-1.7,27.1-1.7,36.3l-1.6,1.6 | ||
| + | c-24.7-0.8-57.2-1.2-97.5-1.2l-57-0.4c-13,0-26.3,0.5-39.8,1.6l-1.6-1.6v-6.4l1.6-2c8.1-4.2,12.5-6.7,13.3-7.6 | ||
| + | c0.8-0.9,1.6-9.3,2.4-25.2c0.8-15.9,1.3-35,1.3-57.4v-83.8l-0.4-38.1c-0.1-14.8-0.5-24.4-1.1-28.7s-1.5-7.2-2.7-8.6 | ||
| + | c-1.2-1.4-3.6-2.6-7.1-3.5c-3.5-0.9-12.2-1.6-26-2.1l-1.6-1.4V32.7z"/> | ||
| + | <path class="st0" d="M1488,308.8l-1.8-1.6v-10.2l1.8-1.6c9.5-0.1,15.6-0.6,18.4-1.5c2.7-0.8,5.2-2.5,7.3-5.1s6.5-10.8,13-24.9 | ||
| + | l20.5-44.5l53.1-120.3c10-23.6,20-47.1,29.9-70.7h12.7l83.4,196.1l11.1,26.2c3.3,7.4,7,15.5,11.2,24.2c4.2,8.7,7.2,13.9,8.8,15.4 | ||
| + | c1.6,1.6,3.6,2.8,6.1,3.6c2.4,0.8,7.8,1.3,16.1,1.5l1.6,1.6v10.2l-1.6,1.6c-13.8-1.2-26.2-1.8-37.3-1.8 | ||
| + | c-21.6,0.7-43.2,1.2-64.8,1.8l-1.6-1.6v-10.2l1.6-1.6c14.6-0.3,23.7-0.9,27.3-1.9c3.6-1,5.5-3.2,5.5-6.7c0-2.9-0.9-6.6-2.7-11.3 | ||
| + | l-24-59.6h-113.1l-21.3,50c-3.8,9-5.7,15.6-5.7,19.7c0,3.3,1.8,5.7,5.3,7.2s12.4,2.4,26.6,2.5l1.8,1.6v10.2l-1.6,1.6 | ||
| + | c-16.8-1-31.5-1.6-44.1-1.6C1517.1,307.3,1502.6,307.8,1488,308.8z M1577.1,199.3h98.8l-49-117.8L1577.1,199.3z"/> | ||
| + | <path class="st0" d="M1797.6,43.8l-1.6-1.4v-9.8l1.6-1.4c22.3,1,39.5,1.6,51.6,1.6c4.6,0,22.6-0.5,54.1-1.6 | ||
| + | c10.2-0.3,17.5-0.4,22.1-0.4c17.6,0,31.5,1.8,41.8,5.3c10.3,3.5,18.6,9.7,24.8,18.7c6.3,8.9,9.4,19,9.4,30.4 | ||
| + | c0,14.5-5.2,27.9-15.6,40.2c-10.4,12.4-27.3,21.2-50.8,26.6c14.1,1.3,25.7,3.2,34.8,5.7c9.1,2.5,17,6.3,23.6,11.4 | ||
| + | c6.6,5.1,11.9,11.8,15.7,20c3.8,8.2,5.8,17.7,5.8,28.5c0,8.5-1.3,17.1-3.9,25.8c-2.6,8.7-7.4,17.4-14.4,26 | ||
| + | c-7,8.6-15.4,16-25.3,22.1c-9.9,6.1-20,10.5-30.4,13.3s-23.2,4.1-38.6,4.1c-3.1,0-12.5-0.3-28.1-1c-17.3-0.4-26.7-0.6-28.1-0.6 | ||
| + | c-8.9,0-19.7,0.5-32.6,1.6l-1.6-1.6v-6.4l1.6-2c7.5-3.4,11.9-5.7,13.1-7c1.2-1.3,2.1-12.8,2.8-34.6c0.7-21.7,1.1-38.3,1.1-49.8 | ||
| + | v-71.7l-0.2-44.3c0-9.6-0.1-17.5-0.4-23.5c-0.3-6.1-0.6-10.4-1.1-13c-0.5-2.6-1.2-4.4-2.2-5.5c-1-1-2.5-1.9-4.4-2.6 | ||
| + | c-1.9-0.7-5.1-1.3-9.7-1.9L1797.6,43.8z M1867.7,146.3c6.5,0.5,13.9,0.8,22.1,0.8c27,0,45.7-4.8,56.3-14.5 | ||
| + | c10.5-9.6,15.8-22.9,15.8-39.6c0-9.8-2-18.1-6.1-24.9c-4-6.8-10.4-12.1-19-15.7c-8.7-3.6-20.4-5.5-35.3-5.5c-12.1,0-22.8,1-32,2.9 | ||
| + | c-1.2,11.7-1.8,32.2-1.8,61.3V146.3z M1868.1,288.7c10,2.3,20.3,3.5,30.9,3.5c18.6,0,33.2-3,43.8-9c10.5-6,18.3-14.3,23.3-24.9 | ||
| + | c5-10.6,7.5-22.2,7.5-34.7c0-18.8-5.3-33.8-15.9-45c-10.6-11.3-33.6-16.9-68.8-16.9c-5.9,0-12.9,0.3-21.1,0.8v66.6L1868.1,288.7z" | ||
| + | /> | ||
| + | |||
| + | </g> | ||
| + | </svg> | ||
| + | |||
| + | |||
| + | |||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
<div class="body"> | <div class="body"> | ||
<div class="parallax"></div> | <div class="parallax"></div> | ||
| − | + | <div class="igem-icon"><a href="https://2018.igem.org/Team:Uppsala"><img src="https://static.igem.org/mediawiki/2018/7/7a/T--Uppsala--WormBusterLogo_White.png"></a></div> | |
| + | <div class= "content blur-box" style="font-size:16px;"> | ||
| − | + | ||
| − | < | + | |
| − | + | ||
| − | + | ||
| − | </ | + | <!-- CONTENT OF WHATS ON THE PAGE --> |
| + | |||
| + | <div id="toc" class="toc"> | ||
| + | <div id="toctitle"></div> | ||
| + | <ul> | ||
| + | <li class="toclevel tocsection"><a href="#Project_Description" class="scroll"> <span id="whereYouAre"> InterLab </span> </a> | ||
| + | <ul> | ||
| + | <li class="toclevel nav-item active"><a href="#Pur" class="nav-link scroll"> Purpose</a></li> | ||
| + | <li class="toclevel nav-item"><a href="#Inter" class="nav-link scroll"> InterLab Study 2018</a></li> | ||
| + | <li class="toclevel nav-item"><a href="#Mat" class="nav-link scroll"> Materials and Methods </a></li> | ||
| + | <li class="toclevel nav-item"><a href="#Exp" class="nav-link scroll"> Our Experience with the InterLab</a></li> | ||
| + | </ul> | ||
| + | </li> | ||
| + | |||
| + | </ul> | ||
</div> | </div> | ||
| − | + | ||
| + | |||
| + | |||
| + | |||
<div class ="content-text" id="scrolldown" > | <div class ="content-text" id="scrolldown" > | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
<div style="height:5em;"></div> | <div style="height:5em;"></div> | ||
<!-- FROM THIS POINT DOWNWARDS YOU START ADDING YOUR STUFF --> | <!-- FROM THIS POINT DOWNWARDS YOU START ADDING YOUR STUFF --> | ||
| − | + | ||
| − | <h1>Purpose</h1> | + | <h1 style="margin-top: 0px;" id="Pur">Purpose</h1> |
<p>Synthetic biology is an emerging and constantly developing field which holds great promise to solve major problems humanity is facing today in terms of biology related medicine. In order to make use of this potential it is very important to reduce measurement variability and ensure there are standards in the field. To contribute towards this, our team has taken part in the iGEM interlab study, which has the noble goal of identifying and correcting the sources of systematic variability in synthetic biology measurement.<br><br> | <p>Synthetic biology is an emerging and constantly developing field which holds great promise to solve major problems humanity is facing today in terms of biology related medicine. In order to make use of this potential it is very important to reduce measurement variability and ensure there are standards in the field. To contribute towards this, our team has taken part in the iGEM interlab study, which has the noble goal of identifying and correcting the sources of systematic variability in synthetic biology measurement.<br><br> | ||
| − | iGEM has in previous interlab studies showed that it is possible to reduce the variability in measurements between labs by measuring GFP expression in absolute fluorescence units which are calibrated against a known concentration of a | + | iGEM has in previous interlab studies showed that it is possible to reduce the variability in measurements between labs by measuring GFP expression in absolute fluorescence units which are calibrated against a known concentration of a fluorescent protein. However, there is still a large source of variability in such measurements depending on the number of cells in the sample. By dividing the total fluorescence by the number of cells we can determine the mean expression level of GFP per cell. Usually, computing the optical density (OD) of the sample gets you an approximation of the number of cells present. OD measurements are however subject to high variability between labs due to spectrophotometer calibrations and is therefore not a precise approximation method. By instead using the direct method of cell count in each sample, one can remove this source of variability in measurements. |
</p> | </p> | ||
| − | <h1>InterLab Study 2018</h1> | + | <h1 id="Inter">InterLab Study 2018</h1> |
<p>By comparing how much the following methods agree with each other we can investigate if by using one approach or both, can help to reduce the variability in measurements between different labs. </p> | <p>By comparing how much the following methods agree with each other we can investigate if by using one approach or both, can help to reduce the variability in measurements between different labs. </p> | ||
| − | <h2>Conversion | + | <h2>Conversion Between Absorbance of Cells to Absorbance of a Known Concentration of Beads</h2> |
| − | <p>By measuring the scattered light from a known concentration of silica beads that are roughly the same size and shape as a normal <i>E.coli</i> cells we converted each | + | <p>By measuring the scattered light from a known concentration of silica beads that are roughly the same size and shape as a normal <i>E.coli</i> cells we converted each lab's absorbance measurement into a standard “equivalent concentration of beads” measurement.</p> |
| − | <h2>Counting | + | <h2>Counting Colony-Forming Units (CFUs) from the Sample</h2> |
| − | <p>By spreading a known concentration of cells in liquid media on a plate to see how many colonies grow, we can determine the cell concentration of the sample as a whole since each colony should grow from one single cell. We determined the number of CFUs in negative and positive control samples to compute a conversion factor from absorbance to CFU. </p> | + | <p>By spreading a known concentration of cells in liquid media on a plate to see how many colonies that grow, we can determine the cell concentration of the sample as a whole since each colony should grow from one single cell. We determined the number of CFUs in negative and positive control samples to compute a conversion factor from absorbance to CFU. </p> |
| − | <h1>Material and Methods</h1> | + | <h1 id="Mat">Material and Methods</h1> |
| − | <h2>Conversion | + | <h2>Conversion Between Absorbance of Cells to Absorbance of a Known Concentration of Beads</h2> |
<p>We made three sets of unit calibration measurements: an OD600 reference point, a particle standard curve, and a fluorescein standard curve.<br><br> | <p>We made three sets of unit calibration measurements: an OD600 reference point, a particle standard curve, and a fluorescein standard curve.<br><br> | ||
| Line 56: | Line 194: | ||
Six different parts from the Distribution Kit along with a positive and negative control (all in a pSB1C3 backbone) were transformed into <i>E. coli</i> K-12 DH5-alpha cells. Fluorescence intensity and OD of the samples was measured with same the instrument and standard settings as the calibrations. Measurements were taken after 0 and 6 hours of liquid culture incubation in 37°C and 220 rpm. </p><br> | Six different parts from the Distribution Kit along with a positive and negative control (all in a pSB1C3 backbone) were transformed into <i>E. coli</i> K-12 DH5-alpha cells. Fluorescence intensity and OD of the samples was measured with same the instrument and standard settings as the calibrations. Measurements were taken after 0 and 6 hours of liquid culture incubation in 37°C and 220 rpm. </p><br> | ||
| − | <p><strong>Table 1 | + | <p><strong>Table 1:</strong> Parts and Devices used. </p> |
| + | <br> | ||
| + | <table class="pgrouptable tablesorter our-table" style="width: 100%;" cellspacing="15" cellpadding="0"> | ||
| − | |||
| + | <thead> | ||
| + | <tr> | ||
| + | <th style= “width: auto”>Part</th> | ||
| + | <th style=“width: auto” >Part Number</th> | ||
| + | |||
| + | </tr> | ||
| + | </thead> | ||
<tbody> | <tbody> | ||
| + | |||
<tr> | <tr> | ||
| − | < | + | <td>Negative Control</td> |
| − | + | <td>BBa_R0040</td> | |
| − | + | ||
| − | </ | + | |
| − | < | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | </ | + | |
</tr> | </tr> | ||
| + | |||
<tr> | <tr> | ||
| − | + | <td>Positive Control</td> | |
| − | + | <td >BBa_I20270</td> | |
| − | <td> | + | |
| − | </td> | + | |
| − | <td> BBa_I20270 | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | </td> | + | |
</tr> | </tr> | ||
| − | |||
| + | <tr> | ||
| + | <td>Test Device 1</td> | ||
| + | <td >BBa_J364000</td> | ||
| + | </tr> | ||
| − | < | + | <tr> |
| − | + | <td>Test Device 2</td> | |
| − | + | <td >BBa_J364001</td> | |
| − | + | </tr> | |
| − | + | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | <tr> | |
| + | <td>Test Device 3</td> | ||
| + | <td >BBa_J364002</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Test Device 4</td> | ||
| + | <td >BBa_J364007</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Test Device 5</td> | ||
| + | <td >BBa_J364008</td> | ||
| + | </tr> | ||
| − | < | + | <tr> |
| − | + | <td>Test Device 6</td> | |
| − | + | <td >BBa_J364009</td> | |
| − | + | </tr> | |
| − | + | ||
| − | + | ||
| − | |||
| − | + | </tbody> | |
| − | + | </table> | |
| − | + | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <!-----------------------HERE---------------------> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <div class="card-holder"> | ||
| + | <div class="content-card-heading"></div> | ||
| + | <div class="content-card content-card-2"> | ||
| + | <div class="inner-card left-card"> | ||
| + | |||
| + | |||
| + | <br> | ||
| + | |||
| + | <!--change src to that of the image you want--> | ||
| + | <img class="content-card-img" src="https://static.igem.org/mediawiki/2018/7/7e/T--Uppsala--Interlab-Fluorescein_Std_Curve.png"> | ||
| + | <div class="inner-card-text"> | ||
| + | <!-- start of paragraph--> | ||
| + | <p><strong>Figure 1.</strong> Fluorescein standard curve based on measured values of fluorescein in serial dilution. Fluorescence intensity was measured with TECAN infinite M200. </p> | ||
| + | </div> | ||
| + | <!-- end of paragraph --> | ||
</div> | </div> | ||
| + | <div class="inner-card right-card"> | ||
| + | |||
| + | <br> | ||
| + | |||
| − | + | <img class="content-card-img" src="https://static.igem.org/mediawiki/2018/5/59/T--Uppsala--Interlab-Particle_Std_Curve.png"> | |
| + | <div class="inner-card-text"> | ||
| + | <!-- start of paragraph --> | ||
| + | <p><strong>Figure 2.</strong> Particle standard curve based on measured absorbance of serial diluted samples of silica beads at a wavelength of 600. | ||
| + | </p> | ||
| + | <!-- End of paragraphs --> | ||
| + | </div> | ||
| + | |||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <!-----------------------HERE---------------------------> | ||
| Line 155: | Line 335: | ||
<br> | <br> | ||
| + | |||
| + | <!--change src to that of the image you want--> | ||
| + | <img class="content-card-img" src="https://static.igem.org/mediawiki/2018/a/a5/T--Uppsala--Interlab-1.jpeg"> | ||
<div class="inner-card-text"> | <div class="inner-card-text"> | ||
<!-- start of paragraph--> | <!-- start of paragraph--> | ||
<p><strong>Figure 3.</strong> Transformation plates containing plasmids with different inserts, from the upper right to lower left: Test Device 4, Colony 2; Test Device 3, Colony 2; Test Device 1, Colony 2; Test Device 2, Colony 2.</p> | <p><strong>Figure 3.</strong> Transformation plates containing plasmids with different inserts, from the upper right to lower left: Test Device 4, Colony 2; Test Device 3, Colony 2; Test Device 1, Colony 2; Test Device 2, Colony 2.</p> | ||
</div> | </div> | ||
| − | |||
| − | |||
<!-- end of paragraph --> | <!-- end of paragraph --> | ||
</div> | </div> | ||
| Line 166: | Line 347: | ||
<br> | <br> | ||
| + | |||
| + | |||
| + | <img class="content-card-img" src="https://static.igem.org/mediawiki/2018/8/8b/T--Uppsala--Interlab-2.jpeg"> | ||
<div class="inner-card-text"> | <div class="inner-card-text"> | ||
<!-- start of paragraph --> | <!-- start of paragraph --> | ||
| Line 171: | Line 355: | ||
<!-- End of paragraphs --> | <!-- End of paragraphs --> | ||
</div> | </div> | ||
| − | |||
| − | |||
| Line 181: | Line 363: | ||
| − | <br><p> All plates were placed under a UV-light to confirm expression of GFP before picking colonies for inoculation. Fluorescent expression can be clearly distinguished from the expected cell culture, see | + | <br><p> All plates were placed under a UV-light (312 nm) to confirm expression of GFP before picking colonies for inoculation. Fluorescent expression can be clearly distinguished from the expected cell culture, see figure 3 and figure 4.</p> |
| − | <h2>Method for | + | <h2>Method for Counting Colony-Forming Units (CFUs) from the Sample</h2> |
| − | <p>To determine the CFU count, we counted colonies from two positive control (<a href="http://parts.igem.org/Part:BBa_I20270"><strong>BBa_I20270</strong></a>) plates and two negative control (<a href="http://parts.igem.org/Part:BBa_R0040"><strong>BBa_R0040</strong></a>) plates. The OD values of the liquid cultures of the four samples were measured and diluted in triplicates to OD = 0.1 in 1 mL of LB + Cam media. Each diluted sample was further diluted in series where samples with dilution factor 8 x 10<sup>-3</sup>, 8 x 10<sup>-4</sup> and 8 x 10<sup>-5</sup> | + | <p>To determine the CFU count, we counted colonies from two positive control (<a href="http://parts.igem.org/Part:BBa_I20270"><strong>BBa_I20270</strong></a>) plates and two negative control (<a href="http://parts.igem.org/Part:BBa_R0040"><strong>BBa_R0040</strong></a>) plates. The OD values of the liquid cultures of the four samples were measured and diluted in triplicates to OD = 0.1 in 1 mL of LB + Cam media. Each diluted sample was further diluted in series where samples with dilution factor 8 x 10<sup>-3</sup>, 8 x 10<sup>-4</sup> and 8 x 10<sup>-5</sup> were streaked on LB + Cam plates.<br><br> |
| − | After | + | After incubation at 37°C for 17 hours the colonies on the plates were counted. Based on the assumption that 1 bacterial cell gives rise to 1 colony, CFU per 1 mL of an OD = 0.1 was calculated by the formula: |
#colonies x Final Dilution Factor = CFU/mL</p> | #colonies x Final Dilution Factor = CFU/mL</p> | ||
| − | <h1>Our | + | <h1 id="Exp">Our Experience with the InterLab Study</h1> |
| − | <p>The interlab is an excellent opportunity to practice cell transformations and streaking of plates. It was also very useful for us since we | + | <p>The interlab is an excellent opportunity to practice cell transformations and streaking of plates. It was also very useful for us since we became more familiar with the iGEM kit plates and how to use the DNA provided in the starting kit. In addition we learned a lot from using the plate reader and the different calibrations, we gathered experience that became useful in other parts of our project.<br><br> |
| − | We were however a | + | We were however a bit confused when reading through the protocols. The protocol for conversion between absorbance of cells to absorbance of a known concentration of beads and the protocol for counting colony-forming units (CFUs) from the sample had different typos which made us believe that it was actually an appendix. In addition, the antibiotic used for selection in the plates (chloramphenicol) was written with different abbreviations between the two protocols; Cam and Chlor. This can be very misleading for an inexperienced student and/or a non-native english speaker. We would therefore recommend using the same abbreviations and formulations in all protocols included in the Interlab Study. In general the cell measurement protocols were quite confusing and could use some attention/optimization. The workflow could have been more clear and better formatted for the usage of a 96 well plate.<br><br> |
| − | This | + | This year's Interlab Study gave us insight in where variability might arise in laboratory work. We strongly recommend all teams to participate in future interlab studies, not only for educational purposes but to also gather experience and become familiar with different measurements and methods. Lastly, it also feels good to be a part of a great interlaboratory project with a meaningful purpose. </p> |
Latest revision as of 14:18, 3 December 2018
Purpose
Synthetic biology is an emerging and constantly developing field which holds great promise to solve major problems humanity is facing today in terms of biology related medicine. In order to make use of this potential it is very important to reduce measurement variability and ensure there are standards in the field. To contribute towards this, our team has taken part in the iGEM interlab study, which has the noble goal of identifying and correcting the sources of systematic variability in synthetic biology measurement.
iGEM has in previous interlab studies showed that it is possible to reduce the variability in measurements between labs by measuring GFP expression in absolute fluorescence units which are calibrated against a known concentration of a fluorescent protein. However, there is still a large source of variability in such measurements depending on the number of cells in the sample. By dividing the total fluorescence by the number of cells we can determine the mean expression level of GFP per cell. Usually, computing the optical density (OD) of the sample gets you an approximation of the number of cells present. OD measurements are however subject to high variability between labs due to spectrophotometer calibrations and is therefore not a precise approximation method. By instead using the direct method of cell count in each sample, one can remove this source of variability in measurements.
InterLab Study 2018
By comparing how much the following methods agree with each other we can investigate if by using one approach or both, can help to reduce the variability in measurements between different labs.
Conversion Between Absorbance of Cells to Absorbance of a Known Concentration of Beads
By measuring the scattered light from a known concentration of silica beads that are roughly the same size and shape as a normal E.coli cells we converted each lab's absorbance measurement into a standard “equivalent concentration of beads” measurement.
Counting Colony-Forming Units (CFUs) from the Sample
By spreading a known concentration of cells in liquid media on a plate to see how many colonies that grow, we can determine the cell concentration of the sample as a whole since each colony should grow from one single cell. We determined the number of CFUs in negative and positive control samples to compute a conversion factor from absorbance to CFU.
Material and Methods
Conversion Between Absorbance of Cells to Absorbance of a Known Concentration of Beads
We made three sets of unit calibration measurements: an OD600 reference point, a particle standard curve, and a fluorescein standard curve.
Six different parts from the Distribution Kit along with a positive and negative control (all in a pSB1C3 backbone) were transformed into E. coli K-12 DH5-alpha cells. Fluorescence intensity and OD of the samples was measured with same the instrument and standard settings as the calibrations. Measurements were taken after 0 and 6 hours of liquid culture incubation in 37°C and 220 rpm.
Table 1: Parts and Devices used.
| Part | Part Number |
|---|---|
| Negative Control | BBa_R0040 |
| Positive Control | BBa_I20270 |
| Test Device 1 | BBa_J364000 |
| Test Device 2 | BBa_J364001 |
| Test Device 3 | BBa_J364002 |
| Test Device 4 | BBa_J364007 |
| Test Device 5 | BBa_J364008 |
| Test Device 6 | BBa_J364009 |
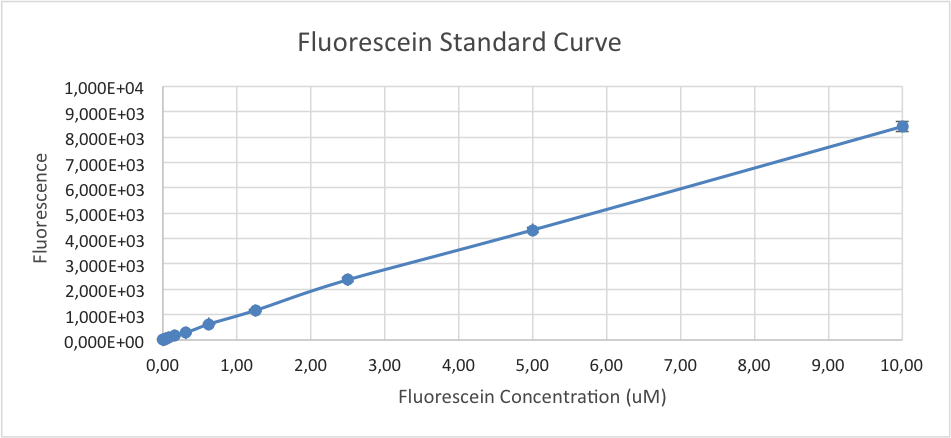
Figure 1. Fluorescein standard curve based on measured values of fluorescein in serial dilution. Fluorescence intensity was measured with TECAN infinite M200.

Figure 2. Particle standard curve based on measured absorbance of serial diluted samples of silica beads at a wavelength of 600.

Figure 3. Transformation plates containing plasmids with different inserts, from the upper right to lower left: Test Device 4, Colony 2; Test Device 3, Colony 2; Test Device 1, Colony 2; Test Device 2, Colony 2.

Figure 4. Transformation plates containing plasmids with different inserts, from the right to left: Test Device 5, Colony 2; Test Device 6, Colony 2.
All plates were placed under a UV-light (312 nm) to confirm expression of GFP before picking colonies for inoculation. Fluorescent expression can be clearly distinguished from the expected cell culture, see figure 3 and figure 4.
Method for Counting Colony-Forming Units (CFUs) from the Sample
To determine the CFU count, we counted colonies from two positive control (BBa_I20270) plates and two negative control (BBa_R0040) plates. The OD values of the liquid cultures of the four samples were measured and diluted in triplicates to OD = 0.1 in 1 mL of LB + Cam media. Each diluted sample was further diluted in series where samples with dilution factor 8 x 10-3, 8 x 10-4 and 8 x 10-5 were streaked on LB + Cam plates.
After incubation at 37°C for 17 hours the colonies on the plates were counted. Based on the assumption that 1 bacterial cell gives rise to 1 colony, CFU per 1 mL of an OD = 0.1 was calculated by the formula:
#colonies x Final Dilution Factor = CFU/mL
Our Experience with the InterLab Study
The interlab is an excellent opportunity to practice cell transformations and streaking of plates. It was also very useful for us since we became more familiar with the iGEM kit plates and how to use the DNA provided in the starting kit. In addition we learned a lot from using the plate reader and the different calibrations, we gathered experience that became useful in other parts of our project.
We were however a bit confused when reading through the protocols. The protocol for conversion between absorbance of cells to absorbance of a known concentration of beads and the protocol for counting colony-forming units (CFUs) from the sample had different typos which made us believe that it was actually an appendix. In addition, the antibiotic used for selection in the plates (chloramphenicol) was written with different abbreviations between the two protocols; Cam and Chlor. This can be very misleading for an inexperienced student and/or a non-native english speaker. We would therefore recommend using the same abbreviations and formulations in all protocols included in the Interlab Study. In general the cell measurement protocols were quite confusing and could use some attention/optimization. The workflow could have been more clear and better formatted for the usage of a 96 well plate.
This year's Interlab Study gave us insight in where variability might arise in laboratory work. We strongly recommend all teams to participate in future interlab studies, not only for educational purposes but to also gather experience and become familiar with different measurements and methods. Lastly, it also feels good to be a part of a great interlaboratory project with a meaningful purpose.

