(→Calibration 3: fluorescence standard curve) |
(→Cell measurements) |
||
| Line 99: | Line 99: | ||
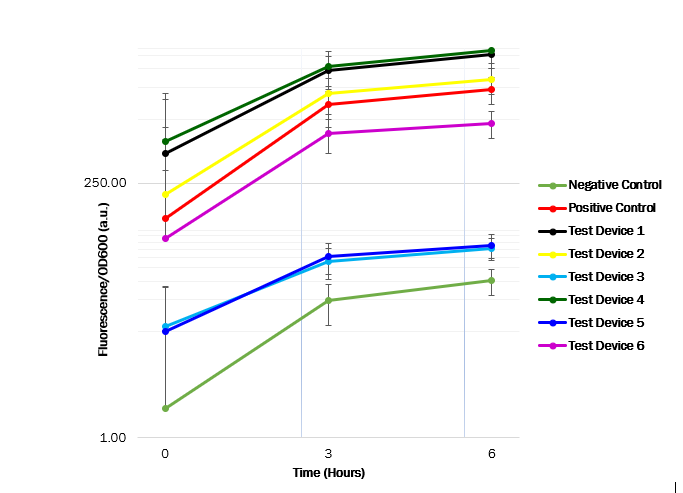
After obtaining OD600 and fluorescence raw readings, the fluorescence per OD ratio was calculated to determine each promotor’s strength. We see that the promoter ([http://parts.igem.org/Part:BBa_J23101 J23101]) in test device 1 ([http://parts.igem.org/Part:BBa_J364000 BBa_J364000]) and the promoter ([http://parts.igem.org/Part:BBa_J23100 J23100]) in test device 4 ([http://parts.igem.org/Part:BBa_J364007 BBa_J364007]) are indeed the strongest promoters, as the GFP expression seems high. However, the promoter ([http://parts.igem.org/Part:BBa_J23106 J23106]) in test device 2 ([http://parts.igem.org/Part:BBa_J364001 BBa_J364001]) and the promoter ([http://parts.igem.org/Part:BBa_J23116 J23116]) in device 6 ([http://parts.igem.org/Part:BBa_J364009 BBa_J364009]) has a medium strength, as the GFP expression was observed to have a medium increase. Whereas the promoter ([http://parts.igem.org/Part:BBa_J23117 J23117]) in test device 3 ([http://parts.igem.org/Part:BBa_J364002 BBa_J364002]) and the promoter ([http://parts.igem.org/Part:BBa_J23104 J23104]) in test device 5 ([http://parts.igem.org/Part:BBa_J364008 BBa_J364008]) showed have a low strength, as the GFP expression showed a low increase. | After obtaining OD600 and fluorescence raw readings, the fluorescence per OD ratio was calculated to determine each promotor’s strength. We see that the promoter ([http://parts.igem.org/Part:BBa_J23101 J23101]) in test device 1 ([http://parts.igem.org/Part:BBa_J364000 BBa_J364000]) and the promoter ([http://parts.igem.org/Part:BBa_J23100 J23100]) in test device 4 ([http://parts.igem.org/Part:BBa_J364007 BBa_J364007]) are indeed the strongest promoters, as the GFP expression seems high. However, the promoter ([http://parts.igem.org/Part:BBa_J23106 J23106]) in test device 2 ([http://parts.igem.org/Part:BBa_J364001 BBa_J364001]) and the promoter ([http://parts.igem.org/Part:BBa_J23116 J23116]) in device 6 ([http://parts.igem.org/Part:BBa_J364009 BBa_J364009]) has a medium strength, as the GFP expression was observed to have a medium increase. Whereas the promoter ([http://parts.igem.org/Part:BBa_J23117 J23117]) in test device 3 ([http://parts.igem.org/Part:BBa_J364002 BBa_J364002]) and the promoter ([http://parts.igem.org/Part:BBa_J23104 J23104]) in test device 5 ([http://parts.igem.org/Part:BBa_J364008 BBa_J364008]) showed have a low strength, as the GFP expression showed a low increase. | ||
| − | [[File:T--Aix-Marseille-- | + | [[File:T--Aix-Marseille--G5.png |600px|center|]] |
These results suggest that the promoter J23101 and J23100 had the strongest affinity for the RNA polymerase when compared with other promoters. Almost all ''E. coli'' strains show a high OD600 value, suggesting a relatively unaffected growth. Thus, the transformed plasmids aren’t toxic to the cells. The table below shows the measured strength of the Anderson promoter collection as displayed in the iGEM registry of standard biological parts. The experimental findings are compatible. | These results suggest that the promoter J23101 and J23100 had the strongest affinity for the RNA polymerase when compared with other promoters. Almost all ''E. coli'' strains show a high OD600 value, suggesting a relatively unaffected growth. Thus, the transformed plasmids aren’t toxic to the cells. The table below shows the measured strength of the Anderson promoter collection as displayed in the iGEM registry of standard biological parts. The experimental findings are compatible. | ||
Revision as of 19:27, 30 August 2018
Interlab
Brief history
Over the past years, iGEM has advanced the frontiers of science with the biggest interlaboratory study ever done in synthetic biology. These studies established a baseline for replicability of fluorescence measurements and identified likely key sources of error and have now been published as an open-access journal article in PLOS ONE.
The InterLab study has been developing a robust measurement procedure for green fluorescent protein (GFP) over the last several years. GFP was chosen as the measurement marker for this study since it's one of the most used markers in synthetic biology and, as a result, most laboratories are equipped to measure this protein.
The fifth interlab study
The fifth international InterLab study aims to identify and correct the sources of systematic variability in synthetic biology measurements so that eventually, measurements that are taken in different labs will be no more variable than measurements taken within the same lab. This year, we helped in answering the following question: Can we reduce lab-to-lab variability in fluorescence measurements by normalizing to absolute cell count or colony-forming units (CFUs) instead of OD?
Materials and methods
Cell measurments
For this study, we transformed six different plasmids, along with a negative and positive control into E. coli K-12 DH5-alpha. The plasmids, from here on referred to as devices, contain a constitutive promoter with low, medium or high strengths. The test devices express GFP (as a reporter in the pSB1C3 backbone). A plasmid containing a constitutive promoter and GFP sequence (BBa_I20270) and a plasmid containing a TetR repressible promoter (BBa_R0040) in the pSB1C3 backbone, were used as positive and negative controls. All plasmids were delivered to us in the iGEM 2018 distribution kit.
We made overnight cultures of two colonies of each transformation plate. The following day, the OD600 values of the overnight cultures were measured, diluted to a target OD600 of 0.02 and grown over 6 hours. Afterward, the absorbance (600 nm) and fluorescence (ex. 485 nm – em. 520 nm, gain = 50) of the growing cultures were measured in a 96 wells black plate in a plate reader (TECAN M200 Infinite) at 0, 3 and 6 hours.
Colony forming units (CFUs)
This procedure can be used to calibrate OD600 to colony forming unit (CFUs) counts, which are directly relatable to the cell concentration of the culture (i.e. viable cell counts per mL). This protocol assumes that 1 bacterial cell will give rise to 1 colony. For the CFU protocol, we counted colonies for our two positive control (BBa_I20270) cultures and our two negative control (BBa_R0040) cultures.
We made overnight cultures. The following day, the OD600 values were measured and diluted to a target OD600 of 0.1. Afterward, we made a dilution series (5 dilutions) with our starting samples. Dilutions 3, 4 and 5 were incubated overnight on LB + agar plates.
Based on the assumption that 1 bacterial cell gives rise to 1 colony, colony forming units (CFU) per 1mL of an OD600 = 0.1 culture can be calculated following two steps: counting the colonies on each plate with fewer than 300 colonies, then multiplying the colony count by the final dilution factor on each plate.
Results
Calibration 1: reference point
We measured Abs600 of LUDOX CL-X and H2O to use them as a reference point to obtain a ratiometric conversion factor. By multiplying this factor with the raw Abs600 measurement data, we could transform our absorbance data into a standard OD600 measurement, accounting for instrument differences.
| LUDOX CL-X | H2O | |
|---|---|---|
| Replicate 1 | 0.0537 | 0.036 |
| Replicate 2 | 0.054099999 | 0.0359 |
| Replicate 3 | 0.056299999 | 0.0361 |
| Replicate 4 | 0.056499999 | 0.0361 |
| Arithmetic Mean | 0.055 | 0.036 |
| Corrected Abs600 | 0.019 | |
| Reference OD600 | 0.063 | |
| OD600/Abs600 | 3.294 |
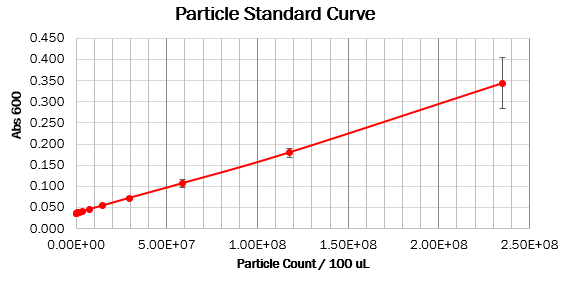
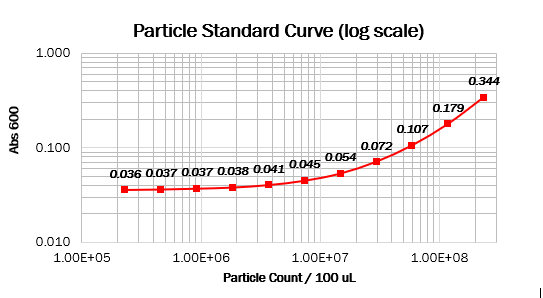
Calibration 2: particle standard curve
We prepared dilution series of monodisperse silica microspheres and measured the Abs600 in our plate reader. The size and optical characteristics of these microspheres are similar to cells, and there is a known amount of particles per volume. This measurement allowed us to construct a standard curve of particle concentration which can be used to convert Abs600 measurements to an estimated number of cells.
Values should form a straight line, with a 1:1 slope, on both linear and log scale curves. The particle standard curve forms a perfect line with a partial 1:1 slope. A possible explanation suggests a consistent pipetting error causing this partial 1:1 slope. Furthermore, the particle standard curve (log scale) doesn’t form a linear curve. To obtain the wanted curve, we should form a baseline for our measurements where we neglect the water’s absorbance at 600 nm.
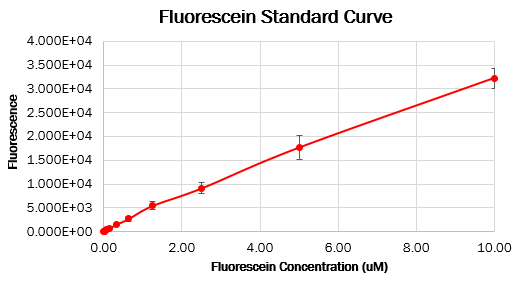
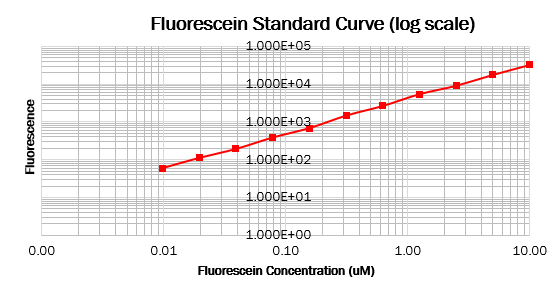
Calibration 3: fluorescence standard curve
To make the standard curve of fluorescence for fluorescein concentration, we made a dilution series of fluorescein in a 96 wells black plate and measured fluorescence with the same settings as the cell measurements. With this standard curve, we could correct our cell measurements to an equivalent fluorescein concentration, ultimately converting this into a concentration of GFP.
Cell measurements
After obtaining OD600 and fluorescence raw readings, the fluorescence per OD ratio was calculated to determine each promotor’s strength. We see that the promoter (J23101) in test device 1 (BBa_J364000) and the promoter (J23100) in test device 4 (BBa_J364007) are indeed the strongest promoters, as the GFP expression seems high. However, the promoter (J23106) in test device 2 (BBa_J364001) and the promoter (J23116) in device 6 (BBa_J364009) has a medium strength, as the GFP expression was observed to have a medium increase. Whereas the promoter (J23117) in test device 3 (BBa_J364002) and the promoter (J23104) in test device 5 (BBa_J364008) showed have a low strength, as the GFP expression showed a low increase.
These results suggest that the promoter J23101 and J23100 had the strongest affinity for the RNA polymerase when compared with other promoters. Almost all E. coli strains show a high OD600 value, suggesting a relatively unaffected growth. Thus, the transformed plasmids aren’t toxic to the cells. The table below shows the measured strength of the Anderson promoter collection as displayed in the iGEM registry of standard biological parts. The experimental findings are compatible.
| Promoter | Measured strength |
|---|---|
| BBa_J23100 | 1 |
| BBa_J23101 | 0.70 |
| BBa_J23104 | 0.72 |
| BBa_J23106 | 0.47 |
| BBa_J23116 | 0.16 |
| BBa_J23117 | 0.06 |
CFUs (Colony Forming Units)
This procedure can be used to calibrate OD600 to colony forming unit (CFUs) counts, which are directly relatable to the cell concentration of the culture (i.e. viable cell counts per mL). This protocol assumes that 1 bacterial cell will give rise to 1 colony. For the CFU protocol, we counted colonies for our two positive control (BBa_I20270) cultures and our two negative control (BBa_R0040) cultures. cultures and our two negative control cultures.
We made overnight cultures. The following day, the OD600 values were measured and diluted to a target OD600 of 0.1. Afterward, we made dilution series (5 dilutions with a 1/10 dilution factor each) with our starting samples. Dilutions 3, 4 and 5 were incubated overnight on LB + agar plates.
Based on the assumption that 1 bacterial cell gives rise to 1 colony, colony forming units (CFUs) per 1mL of an OD600 = 0.1 culture can be calculated following two steps: counting the colonies on each plate with fewer than 300 colonies, then multiplying the colony count by the final dilution factor on each plate.
For the 8 x 10^4 dilution factor plates, all of them had too many colonies to count (over 400 colonies). This is considered "too numerous to count" (TNTC). Whereas, for the 8 x 10^5 dilution and the 8 x 10^6 factor plates, all of them had countable colonies.
| Plate | 8 x 10^4 dilution | 8 x 10^5 dilution | 8 x 10^6 dilution | CFU/mL |
|---|---|---|---|---|
| 1.1 | TNTC | 440 | 59 | 4.72 x 10^8 |
| 1.2 | TNTC | 430 | 49 | 3.92 x 10^8 |
| 1.3 | TNTC | 413 | 44 | 3.52 x 10^8 |
| 2.1 | TNTC | 400 | 46 | 3.68 x 10^8 |
| 2.2 | TNTC | 324 | 40 | 3.2 x 10^8 |
| 2.3 | TNTC | 388 | 52 | 4.16 x 10^8 |
| 3.1 | TNTC | 433 | 41 | 3.28 x 10^8 |
| 3.2 | TNTC | 422 | 44 | 3.52 x 10^8 |
| 3.3 | TNTC | 294 | 55 | 4.4 x 10^8 |
| 4.1 | TNTC | 241 | 21 | 4.72 x 10^8 |
| 4.2 | TNTC | 362 | 30 | 1.68 x 10^8 |
| 4.3 | TNTC | 289 | 32 | 2.56 x 10^8 |
Almost all E. coli strains, with the positive and negative controls plasmids, show an identical CFU, suggesting a relatively unaffected growth. Thus, high expression of the GFP protein isn’t toxic to the cells.
Extra credit: flow cytometry
For extra credit, we collected and submitted flow cytometry data as well. We used the BIO-RAD S3e Cell Sorter for our measurements.
What are the advantages of flow cytometry?
Flow cytometry (FCM) is a technique used for counting and examining individual microscopic particles such as cells based on their fluorescence. One of its unique features is that it measures fluorescence per cell or particle, contrasting with spectrophotometry which measures absorption and transmission of wavelengths as a bulk volume of the sample.
How does flow cytometry work?
The sample is injected into the center of the sheath stream of flow cytometer in a liquid state; therefore, the particles are distributed randomly. The fluidics system is then responsible for separating out the particles into an ordered stream of single particles.
After hydrodynamic focusing, the cells of interest pass through the laser beam, therefore, intercepting and scattering the light which excites the fluorochromes to a higher energy state. The energy is then released as a photon of light with spectral properties unique to specific fluorochromes. Light scattered in the forward direction (as shown in the below diagram) is collected by a lens which is in line with the beam known as the forward scatter channel (FSC). The FSC intensity gives the particles size and can give information used to distinguish between cellular debris and living cells. The side scatters channel (SSC) is perpendicular to the beam and provides information about the granular content within a particle. Both FSC and SSC are unique for each particle and a combination of the two may be used to differentiate between different cell types in a heterogeneous sample.
How does cell sorting work?
The rate of flow sorting at 10 000 cells/second provides a method for sorting a heterogeneous mixture of biological cells into separate storage containers. It is based upon the specific light scattering and fluorescent characteristics of each cell. It is an extremely useful scientific instrument, as it provides a fast, objective and quantitative recording of fluorescent signals from individual cells as well as physical separation of cells of interest.
After the cells have passed through the laser beam and the detectors, a vibrating mechanism causes the stream of cells to break into individual droplets. An electrical charge is placed at the point where the stream breaks into droplets immediately prior to the fluorescence intensity measurement, and the opposite charge is trapped on the droplet as it breaks from the stream. The droplets then travel through a strong electrostatic field and are deflected based on their charge into awaiting sample tubes. The number of cells and level of fluorescence in each tube is then known.
Data collection
Flow cytometers return data as density plots. In this plot, the particle counts are shown by dot density. Each cell recorded is referred to as an event. The green color represents a larger number of events and the red one even more. The different colors are used to create a three-dimensional feel.
The graph shown below is an example of a single-parameter histogram obtained from the flow cytometry during our measurements. These graphs display a single measurement parameter; the relative fluorescence (as shown above) or light scatter intensity on the x-axis and the number of events (cell count) on the y-axis. This graph is very useful for evaluating the total number of cells in a sample that have the physical properties selected for or which express the marker of interest (as is the case with our project). The graph involves flow analysis on a mixed population of cells (some expressing GFP and some are not) this results in several peaks on the histogram. To identify the positive dataset, a positive and a negative control is used for positive identification of the peak corresponding to the cells which were, and which were not expressing GFP.
(Histogram)
Data analysis
Preparations for the flow cytometry measurements
After we collected data from our TECAN (OD600 and fluorescence), we washed and resuspended our samples in 1 mL of PBS to stop the cell growth, thus maintaining the measured OD600 and GFP fluorescence (Samples were on the ice for transportation). We used PBS (1X) and E. coli K-12 DH5-alpha with no plasmids as measurement blanks.