Kristinazu (Talk | contribs) |
|||
| Line 23: | Line 23: | ||
<div class="modal-content"> | <div class="modal-content"> | ||
<section class="design_subsections"> | <section class="design_subsections"> | ||
| − | + | <h1 id="Liposomes">Liposomes</h1> | |
| − | + | <div class="third_level_links"> | |
| − | + | <a href="#Liposomes">Liposomes</a> | |
| − | + | <a href="#Ribosome_modifications">Ribosome modifications</a> | |
| − | + | <a href="#BAM complex">BAM complex</a> | |
| − | + | <a href="#RNA_Thermoswitches">RNA Thermoswitches</a> | |
| − | + | <a href="#Mistic_fusion_protein">Mistic fusion protein</a> | |
| − | + | <a href="#Surface_display_system">Surface display system</a> | |
| − | + | </div> | |
| − | + | <div> | |
| − | + | <h2>Liposomes as closed containers for bottom up research</h2> | |
| − | + | <p></p> | |
| − | + | <p> | |
| − | + | At the core of SynDrop lays a liposome. Liposomes are essentially synthetic vesicles, artificially synthesized droplets of liquid, separated from the environment by a lipid bilayer (Fig.1). They act as containers that encapsulate purified transcriptional and translational machinery and other vital elements that enable complex circuitry design. They have become increasingly popular due to various applications such as being carriers for medicinal drugs<sup>1</sup>, closed environments for protein engineering<sup>2</sup> and characterization of RNAs<sup>3</sup>, as biosensors<sup>4</sup> and molecular diagnostic tools<sup>5</sup>. The growing perspectives of liposomes as scaffolds for synthetic circuitry and membrane protein research are compelling as they have a multitude of different parameters that can be controlled. These include size, composition of a lipid membrane and interior composition. | |
| − | + | </p> | |
| − | + | <p> | |
| − | + | <strong>Fig 1</strong> The composition of a liposome with encapsulated machinery for membrane protein | |
| − | + | integration. Size, membrane composition and interior composition can be easily varied. | |
| − | + | </p> | |
| − | + | <p></p> | |
| − | + | <h2> | |
| − | + | Requirements for liposomes | |
| + | </h2> | ||
| + | <p></p> | ||
| + | <p> | ||
| + | There are many different ways to synthesize liposomes, resulting in a vast range of diverse outputs. For our project, we set particular requirements to suit our scientific design. Ideally, our liposomes must be stable, cell-sized, monodisperse, have a single bilayered membrane and demonstrate excellent encapsulation efficiency. The only approach to optimally combine these attributes proved to be microfluidics. In recent years, droplet-based microfluidic techniques have proved to achieve a robust control over physical parameters of the final product, therefore showing superiority compared to the macroscale bulk methods<sup>6</sup>. We successfully customized and integrated a novel octanol-assisted liposome assembly (OLA)<sup>7</sup> method for high-throughput production of our liposomes. | ||
| + | </p> | ||
| + | <p></p> | ||
| + | <h2> | ||
| + | AutoCAD design for creating photomasks | ||
| + | </h2> | ||
| + | <p></p> | ||
| + | <p> | ||
| + | The first step we had to take towards the production of liposomes was to design, fabricate and prepare a unique microfluidic device. A microfluidic channel design was created with the AutoCAD platform. The prototype acts as a photomask during the photolithography to create a master for the fabrication of microfluidic chips. Our design consists of an array of 16 separate microfluidic channel devices distributed parallelly in groups of four on a single chip (Fig. 2). The dimensions of the microchannels limit the size range of the synthesized liposomes. To dig deeper into the details of how the dimensions of the microfluidic channels influence liposome size we created a phase-field based <strong><var><a href="https://2018.igem.org/Team:Vilnius-Lithuania/Model#COMSOL_model">SynFlow</a></var></strong> model with COMSOL Multiphysics. Auxiliary parametric sweeps were performed that defined the dimensions needed to attain cell – sized liposomes, a range from 5 µm to 30 µm. The final design can be downloaded here and used by anyone interested in the synthesis of liposomes. | ||
| + | </p> | ||
| + | <p><strong>Fig. 2 a</strong> AutoCAD design for the photomask. There are 16 individual microchannel devices on a | ||
| + | single chip. <strong>b</strong> One device consists of three inlets, an outlet and a star-shaped junction.</p> | ||
| + | <p></p> | ||
| + | <h2>Photolithography as a tool for microfluidic chip fabrication</h2> | ||
| + | <p></p> | ||
| + | <p> | ||
| + | After calculating the exact parameters for microfluidic channels and receiving a printed photomask, photolithography is performed to create a master for microfluidic chip preparation. After completing this step, PDMS (<var>polydimethylsiloxane</var>) is poured on to the master left in a thermostat overnight. Inlets and outlets are punched with a biopsy puncher, and the PDMS is cleaned and plasma treated before attaching it to the PDMS coated microscope glass slides. Fig. 3 presents a simplified scheme demonstrating photolithography and other +6steps towards creating a microfluidic chip. To learn more details about the fabrication process, refer to <a href="https://2018.igem.org/Team:Vilnius-Lithuania/Protocols">our Protocols</a>. We called our chip LipoDrop. The final form of LipoDrop is shown in Fig. 4. | ||
| + | </p> | ||
| + | <p> | ||
| + | <strong>Fig. 3</strong> Simplified scheme for microfluidic device preparation. <strong>a-b</strong> the silicon wafer is cleaned and spin-coated with photoresist; <strong>c</strong> the photomask is aligned on the sample and exposed to UV light. <strong>d</strong> sample is submerged to a developer – only the sections that were exposed to the UV light remain intact on the wafer; <strong>e</strong> PDMS is poured onto the master to create a PDMS mold and left for a bake in the oven; <strong>f</strong> the mold is then separated and prepared further by cleaning and punching inlets and outlets; <strong>e-f</strong> a microscopic slide is prepared by applying a thin layer of PDMS on top; <strong>i</strong> PDMS mold and PDMS covered microscopic slide are plasma treated and connected to each other to produce a final microfluidic chip. | ||
| + | </p> | ||
| + | <p> | ||
| + | <strong>Fig. 4</strong> Final form of Lipodrop. | ||
| + | </p> | ||
| + | <p></p> | ||
| + | <h2>Coating LipoDrop with PVA</h2> | ||
| + | <p></p> | ||
| + | <p> | ||
| + | Final and critical step in preparing the chip is the selective coating of post-junction channels with PVA (<var>Polyvinyl alcohol</var>) to render them hydrophilic. It is required to prevent the lipid/octanol solution from wetting the inherently hydrophobic inner channel surface of the device. Without this additional coating, liposomes are unable to form. To coat only a part of microfluidic channels is a challenge: PVA must only remain in post-junction channels without any leakage to the other side. To counter the spreading of PVA to pre-junction channels, air is introduced from separate inlets. A correct interphase between air and PVA must form and stay stable for at least several minutes to let the PVA molecules adhere to the surface (Fig. 5). Any PVA contamination to the opposite side makes the whole device unusable. Not only is this process time consuming (at least 15-20 minutes for each microfluidic device) and requires constant supervision, the procedure quite often fails due to human error while controlling the infusion rates of air and PVA. | ||
| + | </p> | ||
| + | <p> | ||
| + | <strong>Fig. 5</strong> A schematic representation of the interphase of air and PVA at the star shaped junction of LipoDrop. | ||
| + | </p> | ||
| + | <p></p> | ||
| + | <h2>Lipovision software for fully automated microfluidic experiments</h2> | ||
| + | <p></p> | ||
| + | <p> | ||
| + | In pursuit to substantially reduce the manual effort in performing coating procedure, we have designed a software called LipoVision. LipoVision software reduces human labor down to the bare minimum and optimizes the coating procedure entirely. It uses an open standard computer library OpenCV at its’ core, detects the events at the interphase and controls the pumps for the accurate infusion rates according to the situation. The LipoVision software is based on Go and available on all operating systems and is accessible to any custom microfluidic experiment. Eventually, we are planning to apply this software for the fully automated synthesis of the liposomes. To learn more about LipoVision, refer to <a href="https://2018.igem.org/Team:Vilnius-Lithuania/Software">Software</a>. | ||
| + | </p> | ||
| + | <p></p> | ||
| + | <h2>The synthesis of liposomes</h2> | ||
| + | <p></p> | ||
| + | <p> | ||
| + | After successfully coating the chips, the devices are finally ready for liposome synthesis. An experiment is conducted utilizing an octanol-assisted liposome assembly (OLA) method. Liposomes are formed when three unique phases (solutions - OA phase, LO phase and IA phase) form a correct interphase at the junction (Fig. 6). The <strong>IA</strong> phase (inner aqueous) occupies the inner part of the liposome and contains an IVTT transcription/translation system, DNA, membrane protein integration machinery, chaperones and salts needed for protein synthesis and integration into the membrane of the liposome. The <strong>LO</strong> phase contains lipids (i.e. DOPC, Cholesterol) and organic solvent (i.e. 2-octanol); it can also contain fluorescent lipids, such as Rh PE, for imaging. This phase introduces all of the components that will form the liposomes’ membrane. Octanol acts as an organic solvent for phospholipids. In the OLA method, initially double emulsions are formed; the excess octanol and lipids dewet and separate from the droplet leaving double-layered liposomes. Octanol removal from liposomes is crucial as the correct bilayer cannot form in excess organic solvent. Lastly, the <strong>OA</strong> phase (outer aqueous) contains surfactants that help stabilize the droplets at the initial formation and propagation through the microfluidic channels. | ||
| + | </p> | ||
| + | <p> | ||
| + | <strong>Fig. 6</strong> A close-up of the phase interface during liposome synthesis; <strong>IA</strong> phase contains elements required for the synthesis | ||
| + | and integration of membrane proteins; <strong>LO</strong> phase consists of octanol and lipids that form a lipid bilayer; OA solution | ||
| + | carries surfactants that stabilize the initial formation and propagation of the droplets along the microfluidic device. | ||
| + | </p> | ||
| + | <p></p> | ||
| + | <h2>Optimized flow rates for high throughput synthesis</h2> | ||
| + | <p></p> | ||
| + | <p> | ||
| + | With this method, we effectively produce monodisperse bilayered liposomes with high throughput. It is important to note that together with the microchannel dimensions, the infusion rates of the different phases also have an impact on the size of the droplets. Additionally, the infusion rates regulate the throughput of liposome production. The already mentioned <strong><var>SynFlow</var></strong> model determines the optimized flow rates for the most stable and highest frequency synthesis. With the right dimensions and flow rates we reach the production rate that is up to 2000 Hz. The results suggest that this method could be successfully adapted for mass production of liposomes. Fig. 7 reveals the slowed down process of droplet formation. | ||
| + | </p> | ||
| + | <p> | ||
| + | <strong>Fig. 7</strong> High throughput formation of cell-sized liposomes. The video is 60x slowed down | ||
| + | </p> | ||
| + | <p></p> | ||
| + | <p> | ||
| + | A simple liposome size frequency distribution was determined with an image analysis software ImageJ. A plugin SpotCaliper was utilized to identify circular objects and measure their diameters (Fig. 8a). Gaussian distribution was fitted to the frequency histogram. Results verify that the size of the liposomes follows the Gaussian distribution (Fig. 8b). It proves that the droplets are highly homogeneous. Average diameter of a liposome (results from a single batch experiment) is around 12 µm, with standard deviation of 0.4 µm which fits our requirements very well. | ||
| + | </p> | ||
| + | <p> | ||
| + | <strong>Fig. 8 a</strong> An automatic detection of droplets with SpotCaliper: the droplets are marked with teal colored circles and the | ||
| + | diameter of each is measured; <strong>b</strong> size frequency distribution histogram fitted to Gaussian distribution (teal fit) proves | ||
| + | the homogeneity of the liposomes; μ=11.853 >µm±0.017 >µm ; SD=0.442 µm ±0.017 µm. | ||
| + | </p> | ||
| + | <p></p> | ||
| + | <h2>Characterization: encapsulation efficiency and internal synthesis</h2> | ||
| + | <p></p> | ||
| + | <p> | ||
| + | To prove the encapsulation efficiency and test whether protein synthesis and folding are feasible within the liposomes we employed green fluorescent protein (GFP). First, plasmid DNA coding GFP (3.0 ng/µL) was encapsulated together with PURE<var>frex</var> 2.0 transcription translation system. The total reaction mixture was 40 µL. The microtubes were kept on ice during the production and collection of the liposomes. After all the reaction mixture was used up, the collected suspension was incubated in 37℃ for six hours. During the incubation time, negative and positive controls were prepared. The negative control contained the same reaction mixture as previously, excluding the DNA. Positive control, on the other hand, contained purified GFP proteins and no included DNA. The outer solution of all samples contained RNAse to ensure that no synthesis occurred outside the droplets (in case some liposomes erupted and released DNA to the outside). After the incubation period, fluorescent microscopy was utilized to reveal the results. Excitation and emission wavelengths of GFP are 488 nm and 510 nm, respectfully, therefore a suitable FITC filter was used. | ||
| + | </p> | ||
| + | <p> | ||
| + | Brightfield images of the synthesized liposomes are presented in Fig. 9a. The same area was then imaged under the FITC filter Fig. 9b, showing prominent fluorescence. It confirms that the liposomes are biocompatible, and synthesis occurs within them successfully . These results, together with positive control (Fig. 9c) validated that the encapsulation efficiency is immensely effective, as all the liposomes exhibit fluorescence signal. Negative control exhibited no measurable fluorescence with FITC filter, as expected (data not shown), confirming that no contamination was present to distort the results. | ||
| + | </p> | ||
| + | <p> | ||
| + | <strong>Fig. 9 a</strong> brightfield image of the liposomes that contain IVTT system and plasmid GFP DNA (after incubation); | ||
| + | scale bar is 10 µm; <strong>b</strong> liposomes imaged with FITC: fluorescence confirms that transcription and translation | ||
| + | reactions occur inside them; scale bar is 10 µm; <strong>c</strong> liposomes containing purified GFP protein: all the | ||
| + | liposomes exhibit fluorescence validating excellent encapsulation efficiency; scale bar is 20 µm. | ||
| + | </p> | ||
| + | <p></p> | ||
| + | <h2>Characterization: unilamellarity validation using α-hemolysin protein pores</h2> | ||
| + | <p></p> | ||
| + | <p> | ||
| + | Most membrane proteins insert, fold and function properly only in unilamellar membranes, therefore unilamellarity is an absolutely critical parameter for studying MPs. To validate that our synthesized liposomes are truly unilamellar, we recruited the pore forming membrane protein α-hemolysin (2 mM) from Staphylococcus aureus. Monomers of α-hemolysin self-assemble to form mushroom-shaped heptameric 1.5 nm wide pores in the phospholipid bilayer. The integration of these proteins make the bilayer selectively permeable to small molecules sized 2 kDa or less<sup>8</sup>. This protein pore was chosen as it can perforate into only one bilayer, therefore a multilamellar membrane would not allow the internal content of the liposome to escape<sup>9</sup>. | ||
| + | </p> | ||
| + | <p> | ||
| + | To test the incorporation of α-hemolysin into the liposome membrane we used a membrane-impermeable fluorescent probe calcein. Calcein is a self quenching fluorophore - a unique molecule that exhibits fluorescence only after it is diluted. That means that if we encapsulate concentrated calcein solution inside of our liposomes, we should observe that while looking through a fluorescence filter they appear darker than the outer solution (Fig. 10a). That happens because a small fraction of liposomes inevitably falls apart, releasing calcein into the outside, thus diluting it and therefore we measure the fluorescence in the outer solution. Hypothetically, inserting α-hemolysin into the liposome membrane will increase the fluorescence of the outer solution to a much greater degree, as the calcein molecules can now leave the liposomes via the α-hemolysin pore. | ||
| + | </p> | ||
| + | <p> | ||
| + | To test this hypothesis, we prepared two sets of liposomes. Lipid composition of liposomes was composed of DOPC and Cholesterol (cholesterol is necessary for the integration of α-hemolysin). Both sets of liposomes were prepared from the same batch microfluidic experiment. The same volume of liposomes was transferred into a solution with α-hemolysin and to an identical solution without the protein. Plate-reader measurements of fluorescence were then recorded, and results analyzed. As expected, in the absence of α-hemolysin (control), some background fluorescence was observed. However, in the solution where α-hemolysin was included, the measured fluorescence was significantly stronger (p < 0.0001 Fig. 10b) compared to the control group. That confirms the successful integration of α-hemolysin pore into the liposome membrane, explaining the increased fluorescence of the measured outer solution. | ||
| + | </p> | ||
| + | <p> | ||
| + | <strong>Fig. 10 a</strong> concentrated calcein encapsulated within liposomes: the outer solution fluoresces as some of the liposomes | ||
| + | inevitably burst releasing calcein into the outside; <strong>b</strong> box plot comparison of the control (without α-hemolysin) and | ||
| + | a group with inserted α-hemolysin; nonparametrical Mann-Whitney U test was used for the statistical evaluation: | ||
| + | the group with α-hemolysin shows statistically significant (p < 0.0001) increase in fluorescence | ||
| + | </p> | ||
| + | <p></p> | ||
| + | <h3>References</h3> | ||
| + | <p> | ||
| + | <ol> | ||
| + | <li> | ||
| + | Torchilin, V. P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4, 145–160 (2005). | ||
| + | </li> | ||
| + | <li> | ||
| + | Shin, J. & Noireaux, V. An E. coli cell-free expression toolbox: Application to synthetic gene circuits and artificial cells. ACS Synth. Biol. 1, 29–41 (2012). | ||
| + | </li> | ||
| + | <li> | ||
| + | Kapoor, M., Burgess, D. J. & Patil, S. D. Physicochemical characterization techniques for lipid based delivery systems for siRNA. Int. J. Pharm. 427, 35–57 (2012). | ||
| + | </li> | ||
| + | <li> | ||
| + | Michener, J. K., Thodey, K., Liang, J. C. & Smolke, C. D. Applications of genetically-encoded biosensors for the construction and control of biosynthetic pathways. Metab. Eng. 14, 212–222 (2012). | ||
| + | </li> | ||
| + | <li> | ||
| + | Pardee, K. et al. Paper-based synthetic gene networks. Cell 159, 940–954 (2014). | ||
| + | </li> | ||
| + | <li> | ||
| + | Carugo, D., Bottaro, E., Owen, J., Stride, E. & Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 6, (2016). | ||
| + | </li> | ||
| + | <li> | ||
| + | Deshpande, S., Caspi, Y., Meijering, A. E. C. & Dekker, C. Octanol-assisted liposome assembly on chip. Nat. Commun. 7, 1–9 (2016). | ||
| + | </li> | ||
| + | <li> | ||
| + | Noireaux, V. & Libchaber, A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl. Acad. Sci. 101, 17669–17674 (2004). | ||
| + | </li> | ||
| + | <li> | ||
| + | Lu, L., Schertzer, J. W. & Chiarot, P. R. Continuous microfluidic fabrication of synthetic asymmetric vesicles. Lab Chip 15, 3591–3599 (2015). | ||
| + | </li> | ||
| + | </ol> | ||
| + | </p> | ||
| + | </div> | ||
| + | </section> | ||
<section class="design_subsections"> | <section class="design_subsections"> | ||
<h1 id="Ribosome_modifications">Ribosome modifications</h1> | <h1 id="Ribosome_modifications">Ribosome modifications</h1> | ||
Revision as of 23:53, 17 October 2018
Design and Results
Results
Cell-free, synthetic biology systems open new horizons in engineering biomolecular systems which feature complex, cell-like behaviors in the absence of living entities. Having no superior genetic control, user-controllable mechanisms to regulate gene expression are necessary to successfully operate these systems. We have created a small collection of synthetic RNA thermometers that enable temperature-dependent translation of membrane proteins, work well in cells and display great potential to be transferred to any in vitro protein synthesis system.


 Fig. 1 Principle of ribosome attachment to the liposome membrane. The ribosome exit tunnel is localized near the membrane, resulting in transmembrane domains of newly synthesized peptides interacting with the membrane, reducing aggregation
Fig. 1 Principle of ribosome attachment to the liposome membrane. The ribosome exit tunnel is localized near the membrane, resulting in transmembrane domains of newly synthesized peptides interacting with the membrane, reducing aggregation
 Fig. 2 Scheme of the genome modification process:
Fig. 2 Scheme of the genome modification process:
 Fig.3 Example of a constructed donor sequence. The sequence of the selected tag is present in primer used for the PCR of the homology arm that encompasses the target subunit. As a result, the tag sequence is fused to the ribosomal subunit gene.
Fig.3 Example of a constructed donor sequence. The sequence of the selected tag is present in primer used for the PCR of the homology arm that encompasses the target subunit. As a result, the tag sequence is fused to the ribosomal subunit gene.
 Fig. 4 PCR of homology arms, and antibiotic resistance genes
Fig. 4 PCR of homology arms, and antibiotic resistance genes
 Fig. 5 Constructed donor DNA sequences. The L29 donor DNA was not further revisited due to time constraints
Fig. 5 Constructed donor DNA sequences. The L29 donor DNA was not further revisited due to time constraints

 Fig. 2 Electrophoresis gel of PCR products: 6 - Sw2, 7 - Sw3, 8 - Sw6, 9 - Sw7, 10 - Sw9, 11 - Sw11.
Fig. 2 Electrophoresis gel of PCR products: 6 - Sw2, 7 - Sw3, 8 - Sw6, 9 - Sw7, 10 - Sw9, 11 - Sw11.
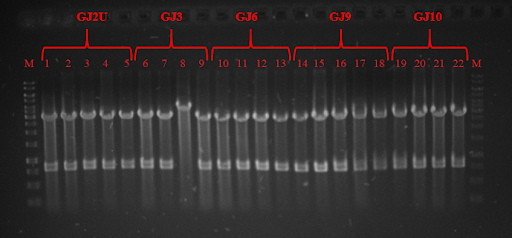 Fig. 3 Restriction analysis of GJx constructs
Fig. 3 Restriction analysis of GJx constructs
 Fig. 4 Colony PCR of RNA thermometers in pSB1C3 plasmid.
Fig. 4 Colony PCR of RNA thermometers in pSB1C3 plasmid.
 Fig. 5 expression at 24 ˚C. On the right you can see GFP expression without RNA thermometer.
Fig. 5 expression at 24 ˚C. On the right you can see GFP expression without RNA thermometer.
 Fig. 6 GFP expression at 30 ˚C. On the right you can see GFP expression without RNA thermometer.
Fig. 6 GFP expression at 30 ˚C. On the right you can see GFP expression without RNA thermometer.
 Fig. 7 GFP expression in 37 ˚C. On the right you can see GFP expression without RNA thermometer.
Fig. 7 GFP expression in 37 ˚C. On the right you can see GFP expression without RNA thermometer.
 Fig. 8 Associational scheme of thermoswitches’ action in the SynDrop system. Not locking the concomitant translation of our target protein and BamA results in target protein aggregation due to insufficient membrane insertion and assembling potential of BamA.
Fig. 8 Associational scheme of thermoswitches’ action in the SynDrop system. Not locking the concomitant translation of our target protein and BamA results in target protein aggregation due to insufficient membrane insertion and assembling potential of BamA.
 Fig. 9 Associational scheme of thermoswitches’ action in the SynDrop system. Locking up translation gives time for proper folding and insertion of BamA and prevents undesirable aggregation of target membrane proteins.
Fig. 9 Associational scheme of thermoswitches’ action in the SynDrop system. Locking up translation gives time for proper folding and insertion of BamA and prevents undesirable aggregation of target membrane proteins.




 Fig. 1 Simplified structure of scFv Antibody
Fig. 1 Simplified structure of scFv Antibody
 Fig. 2 Scheme of scFv_antiVLY and VLY interaction. Left- scFv_antiVLY binds to VLY, erythrocytes stay intact, Right- scFv_antiVLY does not bind and VLY lyse erythrocytes.
Fig. 2 Scheme of scFv_antiVLY and VLY interaction. Left- scFv_antiVLY binds to VLY, erythrocytes stay intact, Right- scFv_antiVLY does not bind and VLY lyse erythrocytes.
 Fig. 3 SDS-PAGE of scFv. GFP is used as positive control, C- chaperone DnaK.
Fig. 3 SDS-PAGE of scFv. GFP is used as positive control, C- chaperone DnaK.
 Fig. 4 Percentage of erythrocyte lysis at different +/-scFv dilutions.
Fig. 4 Percentage of erythrocyte lysis at different +/-scFv dilutions.
 Fig. 5 A- MstX-scFv_antiVLY expression in Escherichia coli. B- scFv_antiVLY and MstX-scFv_antiVLY expression in cell-free system.
Fig. 5 A- MstX-scFv_antiVLY expression in Escherichia coli. B- scFv_antiVLY and MstX-scFv_antiVLY expression in cell-free system.
 Fig. 6 Fig 6. Percentage of erythrocyte lysis at different scFv/MstX-scFv dilutions.
Fig. 6 Fig 6. Percentage of erythrocyte lysis at different scFv/MstX-scFv dilutions.