|
|
| Line 6: |
Line 6: |
| | <div class="col-lg-12"> | | <div class="col-lg-12"> |
| | <div class="story-content"> | | <div class="story-content"> |
| − | <h2 id="scroll-to"><span>Safety</span></h2> | + | <h2 id="scroll-to">The <span>Results</span></h2> |
| − | <p>Working a team of eight people in a laboratory is a very nice experience, very enriching, fun and, above all, it must be very safe. Since the project was raised we were clear that we are a High School team and that the important thing is the protection. Therefore, decisions were made about the design of the project to reduce possible risks to the maximum, how to choose on-pathogenic microorganisms and use all the necessary protective material.</p> | + | <h3 class="about-title mb-70">1. Plasmid construction using GoldenBraid technology</h3> |
| − | <p>Our project is based on developing a method for the purification of recombinant proteins in plants, simple and profitable, which has been developed with the Carbohydrate Metabolism group (belonging to the CSIC) in its laboratories at the Institute of Agrobiotechnology.</p> | + | <p>In order to build our plasmid, we inserted all the parts that we needed in an empty vector using GoldenBraid technology. The transcriptional unit that we have introduced in our plasmids is formed by the 35S promoter (p35S), the Granule-bound starch synthase (GBSS), a factor Xa cleavage site, our protein of interest in this case the GFP and a terminator (Tnos) (Figure 1).</p> |
| − | <p>The laboratory where it has been developed the project, has a level of biosafety 1 (low risk). In addition, the organisms with which we work do not present a potential risk.</p> | + | <div class="text-center mb-20 mb-20"> |
| − | <p>However, during the entire time we were in the laboratory, we met the following security measures:</p> | + | <img src="https://static.igem.org/mediawiki/2018/8/8f/T--Navarra_BG--results_fig1.jpg" alt="Figure 1. Plasmid including the transcriptional unit GBSS-Xa-GFP." class="max_height350"> |
| | + | <span class="pie"><strong>Figure 1</strong>. Plasmid including the transcriptional unit GBSS-Xa-GFP.</span> |
| | + | </div> |
| | + | |
| | + | <h3 class="about-title mb-70">2. Transient transformation of <em>Nicotiana benthamiana</em> with the plasmid pGBSS-Xa-GFP.</h3> |
| | + | <p>After plant transformation we analyzed the present of GFP fluorescent in <em>Nicotina benthamiana</em> leaves using confocal microscopy. As shown in Figure 2 GFP was present in starch granules.</p> |
| | + | <div class="text-center mb-20 mb-20"> |
| | + | <img src="https://static.igem.org/mediawiki/2018/4/43/T--Navarra_BG--results_fig2.jpg" alt="Figure 2. Confocal microscopy of Nicotina benthamiana leaves expressing GBSS-Xa-GFP." class="max_height350"> |
| | + | <span class="pie"><strong>Figure 2</strong>. Confocal microscopy of <em>Nicotina benthamiana</em> leaves expressing GBSS-Xa-GFP.</span> |
| | + | </div> |
| | + | |
| | + | <h3 class="about-title mb-70">3. Starch purification.</h3> |
| | + | <p><em>Nicotiana benthamiana</em> transformed leaves were blended, filtrated and centrifuged (Figure 3) to obtain pure starch in a simple, cheap and high efficient way.</p> |
| | + | <div class="text-center mb-20 mb-20"> |
| | + | <img src="https://static.igem.org/mediawiki/2018/5/53/T--Navarra_BG--results_fig3.jpg" alt="Figure 3. Starch purification steps." class="max_height350"> |
| | + | <span class="pie"><strong>Figure 3</strong>. Starch purification steps.</span> |
| | + | </div> |
| | + | |
| | + | <h3 class="about-title mb-70">4. Release of GFP from starch granules.</h3> |
| | + | <p>Pure starch granules were treated with Xa protease to release free GFP. After proteolytic digestión with Xa protease and a brief centrifugation free GFP was found in the supernatant, while GBSS-GFP remained in pellet fraction as we can see in a western blot using GFP specific antibodies (Figure 4).</p> |
| | + | <div class="text-center mb-20 mb-20"> |
| | + | <img src="https://static.igem.org/mediawiki/2018/8/81/T--Navarra_BG--results_fig4.jpg" alt="Figure 4. Western blot to detect the presence of GFP." class="max_height350"> |
| | + | <span class="pie"><strong>Figure 4</strong>. Western blot to detect the presence of GFP.</span> |
| | + | </div> |
| | | | |
| − | <h3 class="about-title mb-70">Supervised <span>work</span></h3> | + | <h3 class="about-title mb-70">5. Quantification of free GFP.</h3> |
| − | <p>At all times we have been supervised by the people in charge of the laboratory, who have been in charge of supervising the actions carried out and guiding us in the appropriate knowledge and techniques.</p> | + | <p>To quantify released GFP in our sample we did a western blot using GFP antibodies. We used known amounts of standard GFP and different volumes of our supernatant. To confirm that GFP was only released when Xa protease was present we included a negative control without protease (Figure 5).</p> |
| − | <p>Before entering the laboratory, we attended mandatory lectures on Health and Biosafety in the laboratory, where they also explained the protocols and actions to follow in case of emergency.</p>
| + | <div class="text-center mb-20 mb-20"> |
| − |
| + | <img src="https://static.igem.org/mediawiki/2018/d/d8/T--Navarra_BG--results_fig5.jpg" alt="Figure 5. Western blot to quantify GFP." class="max_height350"> |
| − | <h3 class="about-title mb-70">Prevention and safety <span>measures</span></h3> | + | <span class="pie"><strong>Figure 5</strong>. Western blot to quantify GFP.</span> |
| − | <p>We receive lab coats (each person with their own gown), safety goggles, respirators and nitrile and / or latex gloves.<br>We had our own workspace, dedicated to the development of our project; and that we always kept clean and tidy.<br>To reduce any type of risk, when we worked, we disinfected the Ethanol work area before and after each use.</p>
| + | </div> |
| − | <p>The laboratory has a biological safety cabinet in which we managed the bacteria to avoid external contamination.<br>Any waste that we produced was deposited in its corresponding container and processed in the regulatory manner.<br>In addition, Ethidium Bromide has been replaced in the laboratory by Midori Green, a compound with many fewer risks than the previous one.</p> | + | <p>We quantified protein bands by densitometry using commercial GFP as control. Our results showed that in this experimental conditions we are able to obtain 0.5 milligrams of pure GFP from 1 kg of leaf fresh weight.</p> |
| − |
| + | |
| − | <h3 class="about-title mb-70">Organisms</h3> | + | |
| − | <p>To choose well the design of the project depended to avoid many possible biological risks. That is why it was decided to work with non-pathogenic organisms, which have no potential risk outside the laboratory, neither for humans, animals or the environment.</p> | + | <h3 class="about-title mb-70">6. Can we improve GFP yield?</h3> |
| − | <p>These organisms are:</p>
| + | <p>If we are able to increase the amount of starch in leaves we will increase GFP production. As it is known that volatile compounds emitted by <em>Alternaria alternata</em> increase the starch content in leaves (Sánchez-López et al., 2016), we decided to analyze the increment of starch production in Arabidopsis plants growing in the presence of adjacent <em>Alternaria alternata</em> (Figure 6).</p> |
| − | <ul>
| + | <div class="text-center mb-20 mb-20"> |
| − | <li><em>Escherichia coli (TOP 10)</em></li>
| + | <img src="https://static.igem.org/mediawiki/2018/0/0e/T--Navarra_BG--results_fig6.jpg" alt="Figure 6. Volatile Compounds emitted by Alternaria alternata increase the starch content in leaves." class="max_height350"> |
| − | <li><em>Agrobacterium tumefaciens (GV3101)</em></li>
| + | <span class="pie"><strong>Figure 6</strong>. Volatile Compounds emitted by <em>Alternaria alternata</em> increase the starch content in leaves.</span> |
| − | <li><em>Alternaria alternata</em></li> | + | </div> |
| − | </ul>
| + | <p><em>Alternaria alternata</em> volatiles increased 10 times the leaf starch content, so a production of about 5 milligrams of pure GFP per 1 kg of leaf fresh weight will be expected using this strategy.</p> |
| − | <p>In addition to develop our project, we need the following plant species:</p>
| + | |
| − | <ul>
| + | |
| − | <li><em>Nicotiana benthamiana</em></li> | + | |
| − | <li><em>Arabidopsis thaliana</em></li>
| + | |
| − | </ul>
| + | |
| − | <p>In conclusion, from the first moment the experts have supervised all the steps in the laboratory. They have a broad experience working whit these organism and they are totally familiar with all experimental procedures used in this project. The organisms that we are going to use in this project have not potential risks outside the lab.</p>
| + | |
| − | <p>From the first moment all the biosafety rules have been fulfilled.</p> | + | |
| − | <p>All the protocols have been made according to good practices, according to the laws and complying with iGEM’s security rules and policies.</p> | + | |
| − | <p>For more information, you can visit both the website with igem regulations:<br><a href="https://2018.igem.org/Safety/Rules" target="_blank">https://2018.igem.org/Safety/Rules</a><br>like the white list here: <a href="https://2018.igem.org/Safety/White_List" target="_blank">https://2018.igem.org/Safety/White_List</a>.</p>
| + | |
| − |
| + | |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 43: |
Line 56: |
| | </div> | | </div> |
| | </section> | | </section> |
| | + | |
| | </html> | | </html> |
| | {{Navarra_BG/footer}} | | {{Navarra_BG/footer}} |
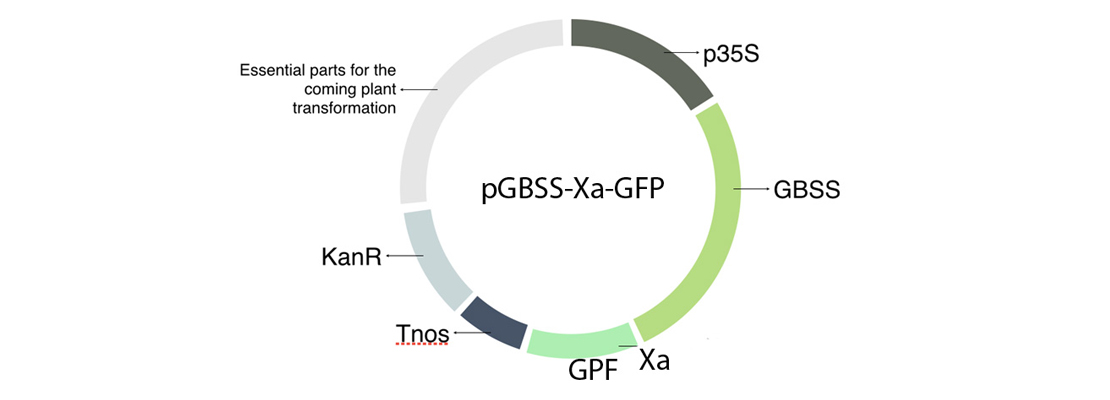 Figure 1. Plasmid including the transcriptional unit GBSS-Xa-GFP.
Figure 1. Plasmid including the transcriptional unit GBSS-Xa-GFP.
 Figure 2. Confocal microscopy of Nicotina benthamiana leaves expressing GBSS-Xa-GFP.
Figure 2. Confocal microscopy of Nicotina benthamiana leaves expressing GBSS-Xa-GFP.
 Figure 3. Starch purification steps.
Figure 3. Starch purification steps.
 Figure 4. Western blot to detect the presence of GFP.
Figure 4. Western blot to detect the presence of GFP.
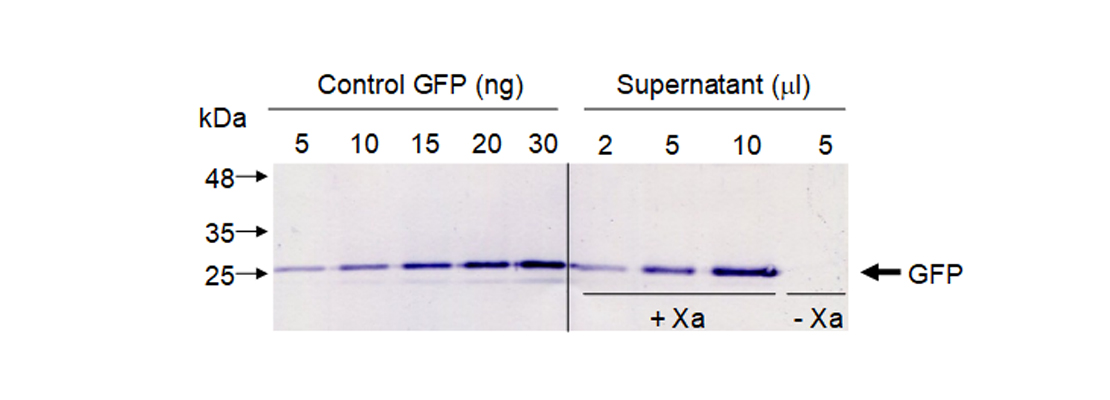 Figure 5. Western blot to quantify GFP.
Figure 5. Western blot to quantify GFP.
 Figure 6. Volatile Compounds emitted by Alternaria alternata increase the starch content in leaves.
Figure 6. Volatile Compounds emitted by Alternaria alternata increase the starch content in leaves.

