The Results
1. Plasmid construction using GoldenBraid technology
In order to build our plasmid, we inserted all the parts that we needed in an empty vector using GoldenBraid technology. The transcriptional unit that we have introduced in our plasmids is formed by the 35S promoter (p35S), the Granule-bound starch synthase (GBSS), a factor Xa cleavage site, our protein of interest in this case the GFP and a terminator (Tnos) (Figure 1).
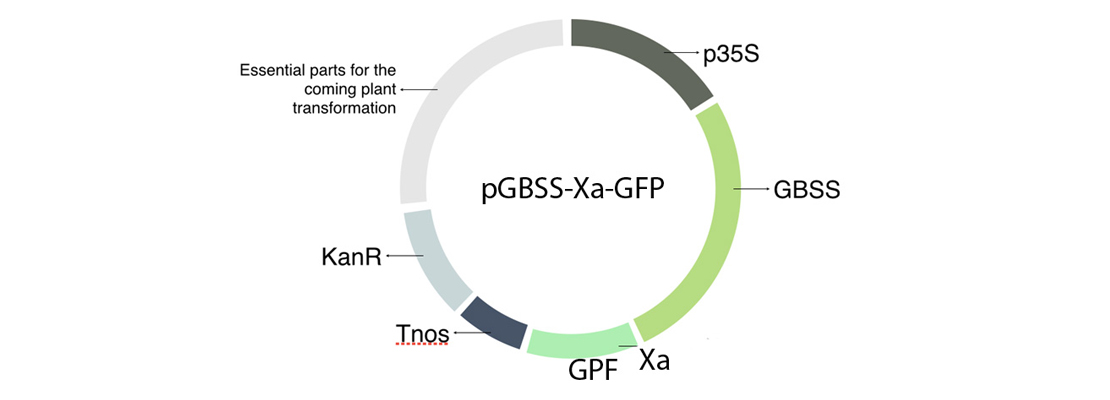 Figure 1. Plasmid including the transcriptional unit GBSS-Xa-GFP.
Figure 1. Plasmid including the transcriptional unit GBSS-Xa-GFP.
2. Transient transformation of Nicotiana benthamiana with the plasmid pGBSS-Xa-GFP.
After plant transformation we analyzed the present of GFP fluorescent in Nicotina benthamiana leaves using confocal microscopy. As shown in Figure 2 GFP was present in starch granules.
 Figure 2. Confocal microscopy of Nicotina benthamiana leaves expressing GBSS-Xa-GFP.
Figure 2. Confocal microscopy of Nicotina benthamiana leaves expressing GBSS-Xa-GFP.
3. Starch purification.
Nicotiana benthamiana transformed leaves were blended, filtrated and centrifuged (Figure 3) to obtain pure starch in a simple, cheap and high efficient way.
 Figure 3. Starch purification steps.
Figure 3. Starch purification steps.
4. Release of GFP from starch granules.
Pure starch granules were treated with Xa protease to release free GFP. After proteolytic digestión with Xa protease and a brief centrifugation free GFP was found in the supernatant, while GBSS-GFP remained in pellet fraction as we can see in a western blot using GFP specific antibodies (Figure 4).
 Figure 4. Western blot to detect the presence of GFP.
Figure 4. Western blot to detect the presence of GFP.
5. Quantification of free GFP.
To quantify released GFP in our sample we did a western blot using GFP antibodies. We used known amounts of standard GFP and different volumes of our supernatant. To confirm that GFP was only released when Xa protease was present we included a negative control without protease (Figure 5).
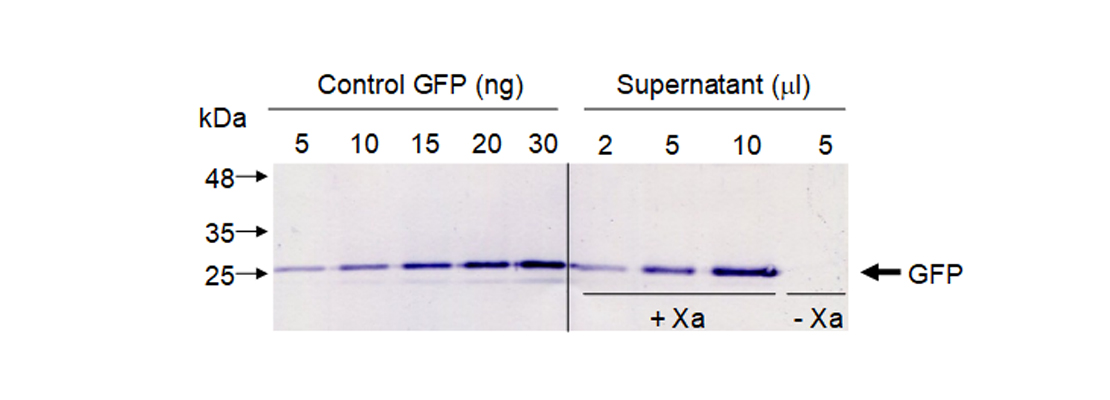 Figure 5. Western blot to quantify GFP.
Figure 5. Western blot to quantify GFP.
We quantified protein bands by densitometry using commercial GFP as control. Our results showed that in this experimental conditions we are able to obtain 0.5 milligrams of pure GFP from 1 kg of leaf fresh weight.
6. Can we improve GFP yield?
If we are able to increase the amount of starch in leaves we will increase GFP production. As it is known that volatile compounds emitted by Alternaria alternata increase the starch content in leaves (Sánchez-López et al., 2016), we decided to analyze the increment of starch production in Arabidopsis plants growing in the presence of adjacent Alternaria alternata (Figure 6).
 Figure 6. Volatile Compounds emitted by Alternaria alternata increase the starch content in leaves.
Figure 6. Volatile Compounds emitted by Alternaria alternata increase the starch content in leaves.
Alternaria alternata volatiles increased 10 times the leaf starch content, so a production of about 5 milligrams of pure GFP per 1 kg of leaf fresh weight will be expected using this strategy.

