|
|
| Line 7: |
Line 7: |
| | <div class="story-content"> | | <div class="story-content"> |
| | <h2 id="scroll-to">The <span>Results</span></h2> | | <h2 id="scroll-to">The <span>Results</span></h2> |
| − | <h3 class="about-title mb-70">1. Plasmid construction using GoldenBraid technology</h3> | + | <ol> |
| − | <p>In order to build our plasmid, we inserted all the parts that we needed in an empty vector using GoldenBraid technology. The transcriptional unit that we have introduced in our plasmids is formed by the 35S promoter (p35S), the Granule-bound starch synthase (GBSS), a factor Xa cleavage site, our protein of interest in this case the GFP and a terminator (Tnos) (Figure 1).</p>
| + | <li> |
| − | <div class="text-center mb-20 mb-20">
| + | <h3 class="about-title mb-70">Plasmid construction using GoldenBraid technology</h3> |
| − | <img src="https://static.igem.org/mediawiki/2018/8/8f/T--Navarra_BG--results_fig1.jpg" alt="Figure 1. Plasmid including the transcriptional unit GBSS-Xa-GFP." class="max_height350">
| + | <p>In order to build our plasmid, we inserted all the parts that we needed in an empty vector using GoldenBraid technology. The transcriptional unit that we have introduced in our plasmids is formed by the 35S promoter (p35S), the Granule-bound starch synthase (GBSS), a factor Xa cleavage site, our protein of interest in this case the GFP and a terminator (Tnos) (Figure 1).</p> |
| − | <span class="pie"><strong>Figure 1</strong>. Plasmid including the transcriptional unit GBSS-Xa-GFP.</span>
| + | <div class="text-center mb-20 mb-20"> |
| − | </div>
| + | <img src="https://static.igem.org/mediawiki/2018/8/8f/T--Navarra_BG--results_fig1.jpg" alt="Figure 1. Plasmid including the transcriptional unit GBSS-Xa-GFP." class="max_height350"> |
| − | | + | <span class="pie"><strong>Figure 1</strong>. Plasmid including the transcriptional unit GBSS-Xa-GFP.</span> |
| − | <h3 class="about-title mb-70">2. Transient transformation of <em>Nicotiana benthamiana</em> with the plasmid pGBSS-Xa-GFP.</h3>
| + | </div> |
| − | <p>After plant transformation we analyzed the present of GFP fluorescent in <em>Nicotina benthamiana</em> leaves using confocal microscopy. As shown in Figure 2 GFP was present in starch granules.</p>
| + | </li> |
| − | <div class="text-center mb-20 mb-20">
| + | <li> |
| − | <img src="https://static.igem.org/mediawiki/2018/4/43/T--Navarra_BG--results_fig2.jpg" alt="Figure 2. Confocal microscopy of Nicotina benthamiana leaves expressing GBSS-Xa-GFP." class="max_height350">
| + | <h3 class="about-title mb-70">Transient transformation of <em>Nicotiana benthamiana</em> with the plasmid pGBSS-Xa-GFP.</h3> |
| − | <span class="pie"><strong>Figure 2</strong>. Confocal microscopy of <em>Nicotina benthamiana</em> leaves expressing GBSS-Xa-GFP.</span>
| + | <p>After plant transformation we analyzed the present of GFP fluorescent in <em>Nicotina benthamiana</em> leaves using confocal microscopy. As shown in Figure 2 GFP was present in starch granules.</p> |
| − | </div>
| + | <div class="text-center mb-20 mb-20"> |
| − | | + | <img src="https://static.igem.org/mediawiki/2018/4/43/T--Navarra_BG--results_fig2.jpg" alt="Figure 2. Confocal microscopy of Nicotina benthamiana leaves expressing GBSS-Xa-GFP." class="max_height350"> |
| − | <h3 class="about-title mb-70">3. Starch purification.</h3>
| + | <span class="pie"><strong>Figure 2</strong>. Confocal microscopy of <em>Nicotina benthamiana</em> leaves expressing GBSS-Xa-GFP.</span> |
| − | <p><em>Nicotiana benthamiana</em> transformed leaves were blended, filtrated and centrifuged (Figure 3) to obtain pure starch in a simple, cheap and high efficient way.</p>
| + | </div> |
| − | <div class="text-center mb-20 mb-20">
| + | </li> |
| − | <img src="https://static.igem.org/mediawiki/2018/5/53/T--Navarra_BG--results_fig3.jpg" alt="Figure 3. Starch purification steps." class="max_height350">
| + | <li> |
| − | <span class="pie"><strong>Figure 3</strong>. Starch purification steps.</span>
| + | <h3 class="about-title mb-70">Starch purification.</h3> |
| − | </div>
| + | <p><em>Nicotiana benthamiana</em> transformed leaves were blended, filtrated and centrifuged (Figure 3) to obtain pure starch in a simple, cheap and high efficient way.</p> |
| − | | + | <div class="text-center mb-20 mb-20"> |
| − | <h3 class="about-title mb-70">4. Release of GFP from starch granules.</h3>
| + | <img src="https://static.igem.org/mediawiki/2018/5/53/T--Navarra_BG--results_fig3.jpg" alt="Figure 3. Starch purification steps." class="max_height350"> |
| − | <p>Pure starch granules were treated with Xa protease to release free GFP. After proteolytic digestión with Xa protease and a brief centrifugation free GFP was found in the supernatant, while GBSS-GFP remained in pellet fraction as we can see in a western blot using GFP specific antibodies (Figure 4).</p>
| + | <span class="pie"><strong>Figure 3</strong>. Starch purification steps.</span> |
| − | <div class="text-center mb-20 mb-20">
| + | </div> |
| − | <img src="https://static.igem.org/mediawiki/2018/8/81/T--Navarra_BG--results_fig4.jpg" alt="Figure 4. Western blot to detect the presence of GFP." class="max_height350">
| + | </li> |
| − | <span class="pie"><strong>Figure 4</strong>. Western blot to detect the presence of GFP.</span>
| + | <li> |
| − | </div>
| + | <h3 class="about-title mb-70">Release of GFP from starch granules.</h3> |
| − |
| + | <p>Pure starch granules were treated with Xa protease to release free GFP. After proteolytic digestión with Xa protease and a brief centrifugation free GFP was found in the supernatant, while GBSS-GFP remained in pellet fraction as we can see in a western blot using GFP specific antibodies (Figure 4).</p> |
| − | <h3 class="about-title mb-70">5. Quantification of free GFP.</h3>
| + | <div class="text-center mb-20 mb-20"> |
| − | <p>To quantify released GFP in our sample we did a western blot using GFP antibodies. We used known amounts of standard GFP and different volumes of our supernatant. To confirm that GFP was only released when Xa protease was present we included a negative control without protease (Figure 5).</p>
| + | <img src="https://static.igem.org/mediawiki/2018/8/81/T--Navarra_BG--results_fig4.jpg" alt="Figure 4. Western blot to detect the presence of GFP." class="max_height350"> |
| − | <div class="text-center mb-20 mb-20">
| + | <span class="pie"><strong>Figure 4</strong>. Western blot to detect the presence of GFP.</span> |
| − | <img src="https://static.igem.org/mediawiki/2018/d/d8/T--Navarra_BG--results_fig5.jpg" alt="Figure 5. Western blot to quantify GFP." class="max_height350">
| + | </div> |
| − | <span class="pie"><strong>Figure 5</strong>. Western blot to quantify GFP.</span>
| + | </li> |
| − | </div>
| + | <li> |
| − | <p>We quantified protein bands by densitometry using commercial GFP as control. Our results showed that in this experimental conditions we are able to obtain 0.5 milligrams of pure GFP from 1 kg of leaf fresh weight.</p>
| + | <h3 class="about-title mb-70">Quantification of free GFP.</h3> |
| − | | + | <p>To quantify released GFP in our sample we did a western blot using GFP antibodies. We used known amounts of standard GFP and different volumes of our supernatant. To confirm that GFP was only released when Xa protease was present we included a negative control without protease (Figure 5).</p> |
| − | | + | <div class="text-center mb-20 mb-20"> |
| − | <h3 class="about-title mb-70">6. Can we improve GFP yield?</h3>
| + | <img src="https://static.igem.org/mediawiki/2018/d/d8/T--Navarra_BG--results_fig5.jpg" alt="Figure 5. Western blot to quantify GFP." class="max_height350"> |
| − | <p>If we are able to increase the amount of starch in leaves we will increase GFP production. As it is known that volatile compounds emitted by <em>Alternaria alternata</em> increase the starch content in leaves (Sánchez-López et al., 2016), we decided to analyze the increment of starch production in Arabidopsis plants growing in the presence of adjacent <em>Alternaria alternata</em> (Figure 6).</p>
| + | <span class="pie"><strong>Figure 5</strong>. Western blot to quantify GFP.</span> |
| − | <div class="text-center mb-20 mb-20">
| + | </div> |
| − | <img src="https://static.igem.org/mediawiki/2018/0/0e/T--Navarra_BG--results_fig6.jpg" alt="Figure 6. Volatile Compounds emitted by Alternaria alternata increase the starch content in leaves." class="max_height350">
| + | <p>We quantified protein bands by densitometry using commercial GFP as control. Our results showed that in this experimental conditions we are able to obtain 0.5 milligrams of pure GFP from 1 kg of leaf fresh weight.</p> |
| − | <span class="pie"><strong>Figure 6</strong>. Volatile Compounds emitted by <em>Alternaria alternata</em> increase the starch content in leaves.</span>
| + | </li> |
| − | </div>
| + | <li> |
| − | <p><em>Alternaria alternata</em> volatiles increased 10 times the leaf starch content, so a production of about 5 milligrams of pure GFP per 1 kg of leaf fresh weight will be expected using this strategy.</p>
| + | <h3 class="about-title mb-70">Can we improve GFP yield?</h3> |
| | + | <p>If we are able to increase the amount of starch in leaves we will increase GFP production. As it is known that volatile compounds emitted by <em>Alternaria alternata</em> increase the starch content in leaves (Sánchez-López et al., 2016), we decided to analyze the increment of starch production in Arabidopsis plants growing in the presence of adjacent <em>Alternaria alternata</em> (Figure 6).</p> |
| | + | <div class="text-center mb-20 mb-20"> |
| | + | <img src="https://static.igem.org/mediawiki/2018/0/0e/T--Navarra_BG--results_fig6.jpg" alt="Figure 6. Volatile Compounds emitted by Alternaria alternata increase the starch content in leaves." class="max_height350"> |
| | + | <span class="pie"><strong>Figure 6</strong>. Volatile Compounds emitted by <em>Alternaria alternata</em> increase the starch content in leaves.</span> |
| | + | </div> |
| | + | <p><em>Alternaria alternata</em> volatiles increased 10 times the leaf starch content, so a production of about 5 milligrams of pure GFP per 1 kg of leaf fresh weight will be expected using this strategy.</p> |
| | + | </li> |
| | + | </ol> |
| | </div> | | </div> |
| | </div> | | </div> |
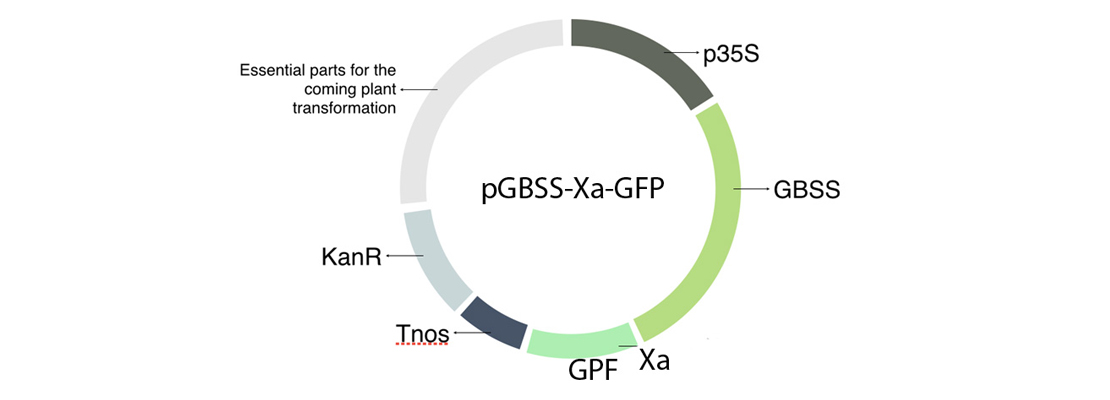 Figure 1. Plasmid including the transcriptional unit GBSS-Xa-GFP.
Figure 1. Plasmid including the transcriptional unit GBSS-Xa-GFP.
 Figure 2. Confocal microscopy of Nicotina benthamiana leaves expressing GBSS-Xa-GFP.
Figure 2. Confocal microscopy of Nicotina benthamiana leaves expressing GBSS-Xa-GFP.
 Figure 3. Starch purification steps.
Figure 3. Starch purification steps.
 Figure 4. Western blot to detect the presence of GFP.
Figure 4. Western blot to detect the presence of GFP.
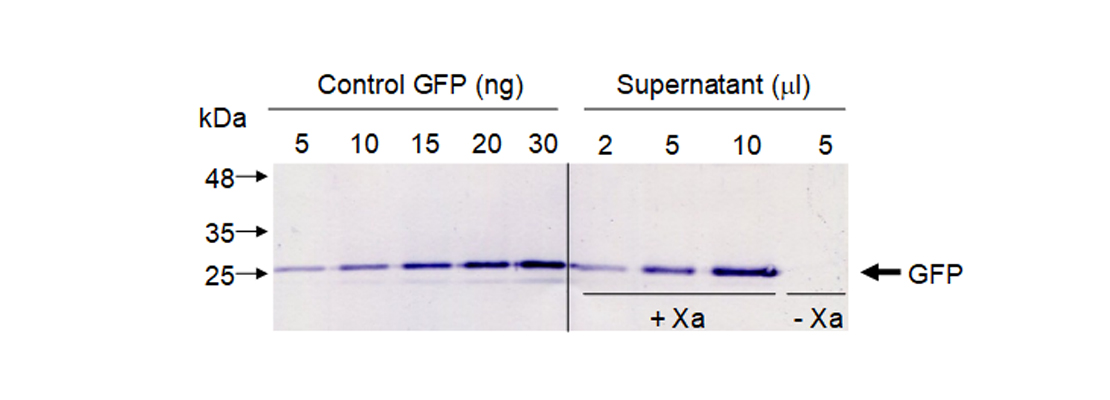 Figure 5. Western blot to quantify GFP.
Figure 5. Western blot to quantify GFP.
 Figure 6. Volatile Compounds emitted by Alternaria alternata increase the starch content in leaves.
Figure 6. Volatile Compounds emitted by Alternaria alternata increase the starch content in leaves.

