{{{title}}}
Results
Here you can describe the results of your project and your future plans.
What should this page contain?
- Clearly and objectively describe the results of your work.
- Future plans for the project.
- Considerations for replicating the experiments.
Describe what your results mean
- Interpretation of the results obtained during your project. Don't just show a plot/figure/graph/other, tell us what you think the data means. This is an important part of your project that the judges will look for.
- Show data, but remember all measurement and characterization data must be on part pages in the Registry.
- Consider including an analysis summary section to discuss what your results mean. Judges like to read what you think your data means, beyond all the data you have acquired during your project.
Project Achievements
You can also include a list of bullet points (and links) of the successes and failures you have had over your summer. It is a quick reference page for the judges to see what you achieved during your summer.
- A list of linked bullet points of the successful results during your project
- A list of linked bullet points of the unsuccessful results during your project. This is about being scientifically honest. If you worked on an area for a long time with no success, tell us so we know where you put your effort.
Inspiration
See how other teams presented their results.
Contents
Results
CHITINASE
Chitinase production:
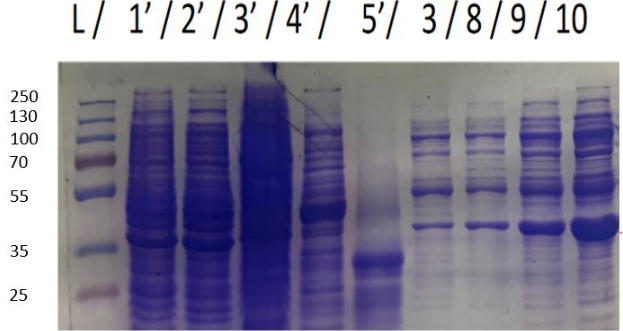
We achieve to produce chitinase thanks to our construction. An inducible overproduction by pLac promoter was used to study chitinase production, induction or none-induction experiments by IPTG can show the presence of the protein. We choose to produce chitinase by E. coli K-12 DH5-alpha strain (and by BL21 strain ?) For this two strains two differents recombinated plasmids with chit1 were tested, plasmid N°10 and N°11.
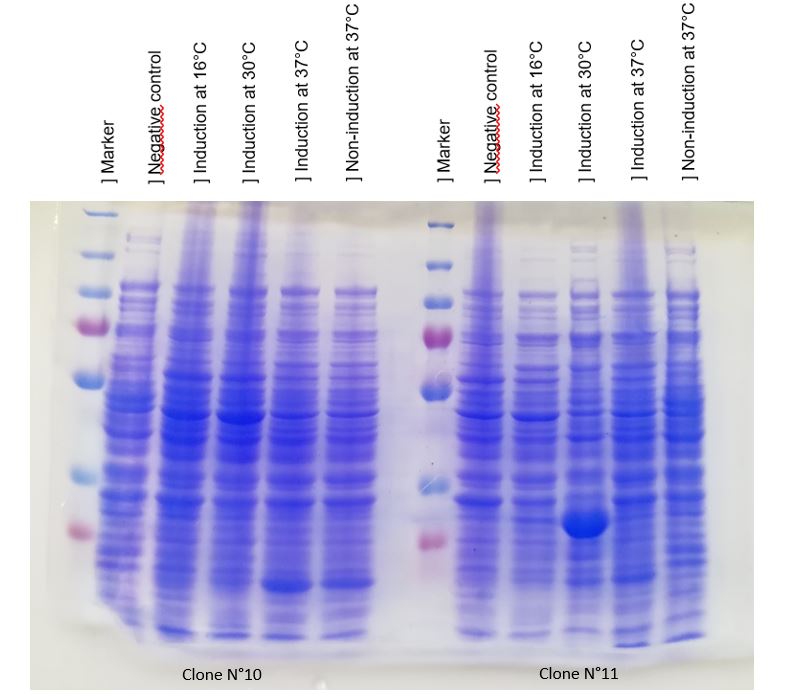
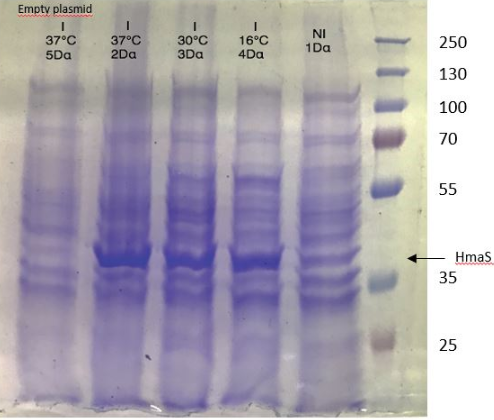
Figure 1: Figure 1: SDS-PAGE of chitinase produced by E. coli K-12 DH5-alpha.
(put markers lenghts on the image!!!! and indicate chitinase band )
As seen in Figure 1, a protein at the expected lenght (30 kDa) was overproduced only at 30°C after IPTG induction in DH5alpha. The non-induction by IPTG show an low band of the protein overproduced. This overproduction should be the chitinase, but it isn't normal that clone N°11 wasn't able to produced at least a significant chitinase quantity at 16°C and 37°C. We clarified that thanks to a western blot, to be sure of chitinase expression by clone N°11.
Chitinase output are better after IPTG induction at 30°C. This condition of culture will be used to produce in large quantity chitinase for purification and activity tests.
(put file of western and blue of BL21 or not?)
Chitinase purification:
Chitinase designed had a polyhistidine-tag which allows it to be specifically separated among total proteins. A Akta purification was realized for purify Chit1. Akta Pure is a automated chromatography system for quick and easy purification. A nickel-nitrilotriacetic acid column was necessary to allow specifical interaction between histidines residues and metal ions from the nickel-nitrilotriacetic acid matrix.
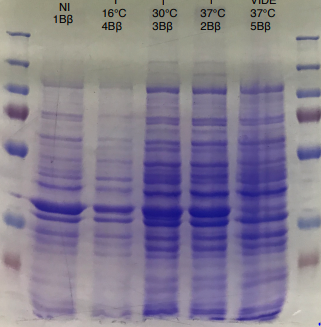
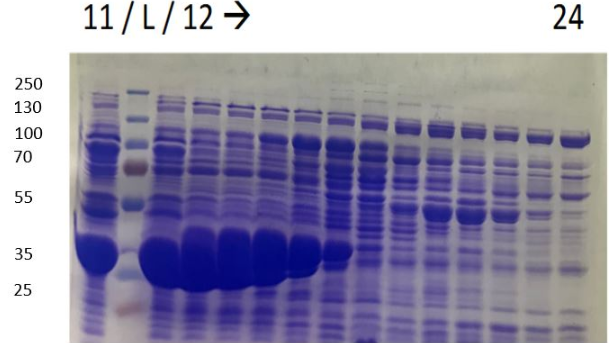
Figure 2: SDS-PAGE of polyhistidine-tagged chitinase purification produced by E. coli K-12 DH5-alpha, using a nickel-nitrilotriacetic acid matrix.
(put markers lenghts on the image!!!! and indicate chitinase band )
Showed in figure 2, a protein at 30 kDa corresponding at the chitinase is obtained on bacteria lysate, on the non-purified fraction and on fraction N°6 to fraction N°10. This fractions can be pooled and used for activity tests.
Numerous controls has been done,chitinase was produced without its peptide signal of secretion, so it will be contained in the cytoplasm, the absence of the band at 30 kDa in the pellet verify that chitinase wasn't contained in the membrane.
Bacteria lysate confirmed the presence of chitinase at 30kDa; non-purified migration shows a low quantity of chitinase with all proteins contaminants; break-though sample didn't contain the protein target demonstrating that all chitinase was fixed on nickel-nitrilotriacetic acid matrix.
The results of chitinase purification prove that chitinase can be purified thanks to its polyhistidine-tag. But purification wasn't optimal and can be ameliorated because a lot of contaminants are isolated with chitinase. The nickel-nitrilotriacetic acid matrix column used was well-worn, that can reduced its efficiency. For an optimal separation of chitinase, we should pool eluate fractions containing chitinase and purify until reduce significantly protein contaminants. But without time we continued with this purification to do activity tests.
Chitinase activity test:
Schales' procedure was used to test the chitinase activity. Schales' procedure is a colorimetric method commoly used for measuring the reducing sugars. Chitin is composed of sugar units of either N-acetyl-G-glucosamine. Chitinase degrade chitin producing N-acetyl-G-glucosamine. To monitor chitinase activity, the reducing sugars released after chitin hydrolyse are detected by a color diminution of the yellow Shales' reagent. This color diminution is translated by a absorbance diminution.
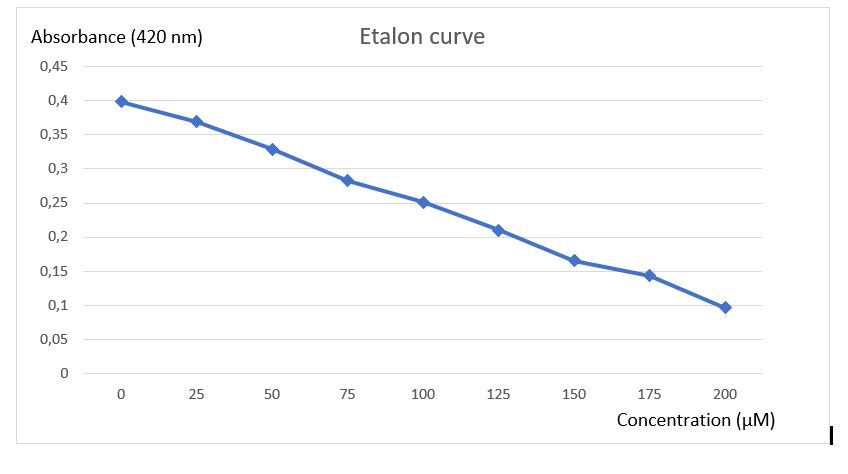
To analyse activity test results, an etalon curve have been done using the final product of chitinase reaction (N-acetyl-G-glucosamine), to know in which concentration of final product must be obtained after reaction to be detected by the method of Schales.
Figure 3: Etalon curve of N-acetyl-G-glucosamine after Schales's activity test.
A final curve has been obtain showing a absorbance diminution in function of increasing N-acetyl-G-glucosamine concentration. With this etalon curve, concentration of final product obtain after chitinase reaction can be determined. This results revealed the efficiency of Schales'procedure to detected N-acetyl-G-glucosamine to 0 at 200 uM.
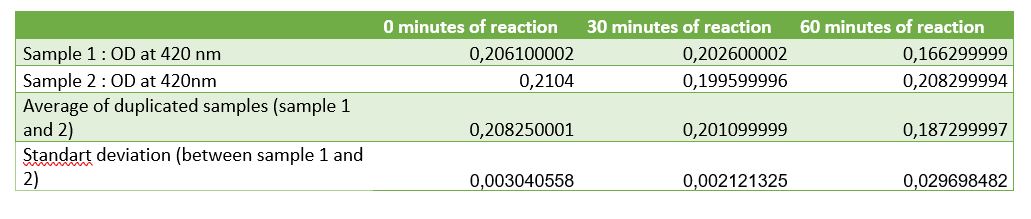
Figure 4: Table of absorbance measurements of chitinase activity test
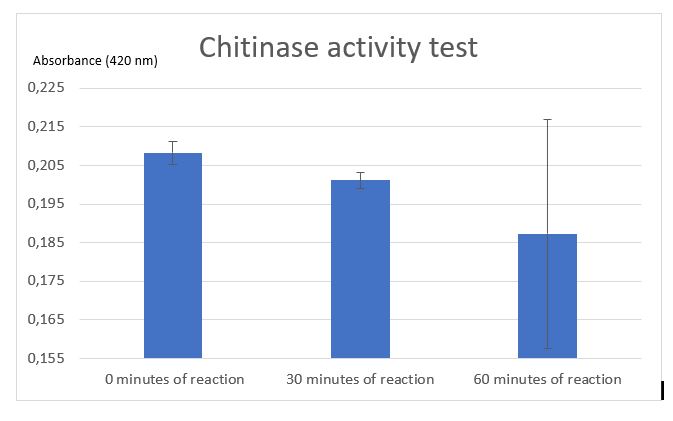
Figure 5: Chitinase activity test by Schales' procedure
According to figure 4, after 30 minutes of reaction a significant activity was mesured. But after 1 hour of reaction the standart deviation put in doubt the significant result at 30 minutes. One of the difficulties encountered was the chitin sample. Chitin sample wasn't completely dissolved in water, it was precipited and a lot of big fragments af shells of shrimps was contained making difficult the pippetage and with difficulty reproducibility. That's why an centrifugation step was added to avoid precipitated chitin to asborbe and affects the results. After centrifugation chitin wasn't really pelleted, it was easily removed. Chitin pellet should be removed in the duplicated sample of 1 hour after reaction, that can be the cause of the high standart deviation in the results. Due to limited time, we weren't able to do another activity test to improve the results and be the most careful at removing and may be we should centrigutated a longer time (not 1 minute but 5 minutes maybe).
Thanks to etalon curve if we consider only the significant results at 30 minutes, we can conclude that after 30 minutes of enzymatic reaction, our chitinase can released 125 uM of N-acetyl-G-glucosamine.
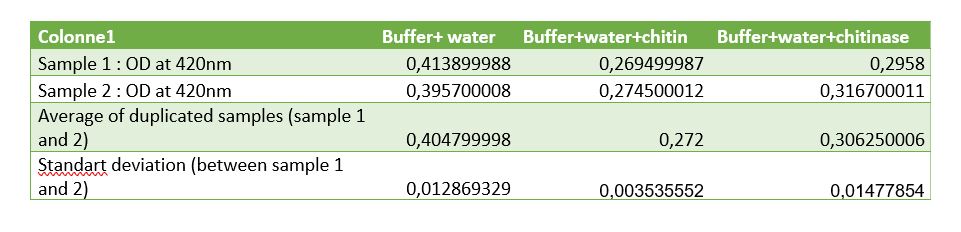
Figure 6: Table of measurements of controls for chitinase activity test
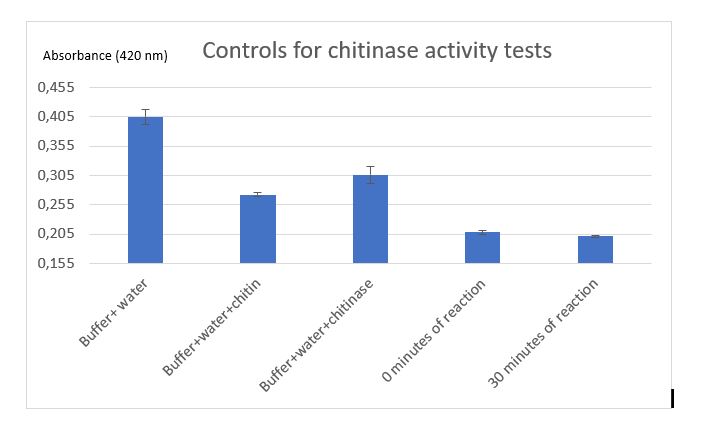
Figure 7: Controls for chitinase activity test
( comment the results of table and the graph!!!!!)
hmaS
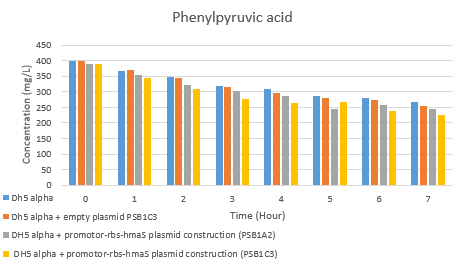
4-hydroxyphenylpyruvate synthase production
4-hydroxyphenylpyruvate synthase purification
4-hydroxyphenylpyruvate synthase activity test
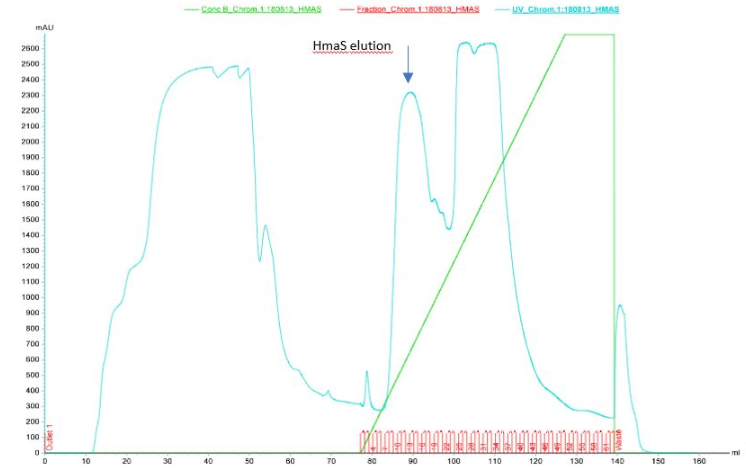
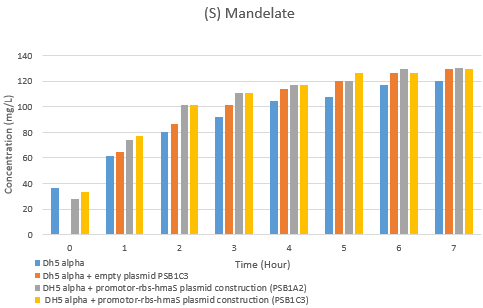
After inserting the p_lac-RBS-hmaS construct into the psb1c3 plasmid [1] and getting our 4-hydroxyphenylpyruvate synthase production and purification results, we were wondered if our protein was functional. In order to test the 4-hydroxyphenylpyruvate synthase activity in our E.coli strain, we have made an HPLC test on supernatent cells as described in our protocols tab : [2]
Results of trap tests
(put results of trap tests on bed bugs)