| (4 intermediate revisions by the same user not shown) | |||
| Line 23: | Line 23: | ||
<div class="drop"> | <div class="drop"> | ||
<p2>Redox molecules are maintained in a reduced form in ambient conditions. Application of a +0.5V potential oxidises the redox molecules, allowing for activation of the genetic circuit. Application of a -0.3V potential ensures the redox molecules remain reduced, preventing activation of the genetic circuit.</p2> | <p2>Redox molecules are maintained in a reduced form in ambient conditions. Application of a +0.5V potential oxidises the redox molecules, allowing for activation of the genetic circuit. Application of a -0.3V potential ensures the redox molecules remain reduced, preventing activation of the genetic circuit.</p2> | ||
| − | <img class="center" src="https://static.igem.org/mediawiki/2018/ | + | <img class="center" src="https://static.igem.org/mediawiki/2018/d/d6/T--Imperial_College--Electrochemicalmodulenew.png" alt="" width="50%";> |
</div> | </div> | ||
<button class="collapsible">Details</button> | <button class="collapsible">Details</button> | ||
| Line 40: | Line 40: | ||
<h4>Ferrocyanide/Ferricyanide:</h4> | <h4>Ferrocyanide/Ferricyanide:</h4> | ||
<p2>These molecules are well known redox mediators, meaning, they alter the redox-state of the cell. When the reduced form (ferricyanide) is present a reducing cellular environment is created, preventing the induction of redox-sensing gene circuits. When the oxidised form (ferrocyanide) is present an oxidising cellular environment is creating, permitting activation of redox-sensing gene circuit. </br><div class="center"> | <p2>These molecules are well known redox mediators, meaning, they alter the redox-state of the cell. When the reduced form (ferricyanide) is present a reducing cellular environment is created, preventing the induction of redox-sensing gene circuits. When the oxidised form (ferrocyanide) is present an oxidising cellular environment is creating, permitting activation of redox-sensing gene circuit. </br><div class="center"> | ||
| − | <img src="https://static.igem.org/mediawiki/2018/3/38/T--Imperial_College--Ferrostructure.png" alt="" width="20%"; > | + | <img src="https://static.igem.org/mediawiki/2018/3/38/T--Imperial_College--Ferrostructure.png" alt="" width="20%"; > |
| − | <img src="https://static.igem.org/mediawiki/2018/1/17/T--Imperial_College--Ferristructure.png" alt="" width="20%"; > | + | <img src="https://static.igem.org/mediawiki/2018/1/17/T--Imperial_College--Ferristructure.png" alt="" width="20%"; ></p2></div> |
<h4>Sodium Sulfite:</h4> | <h4>Sodium Sulfite:</h4> | ||
| Line 47: | Line 47: | ||
Supposed Electrochemical Module Mechanism: | Supposed Electrochemical Module Mechanism: | ||
Sulfite removes oxygen from solution allowing pyocyanin to be maintained in a reduced state. A potential of +0.5V generates oxidised pyocyanin and ferricyanide. Ferricyanide pushes the cell into an oxidising condition, allowing pyocyanin to remain oxidised and activate gene expression of a redox sensing gene circuit. A -0.3V potential generated reduced pyocyanin and ferrocyanide. Ferrocyanide pushes the cell into a reducing condition, allowing pyocyanin to remain reduced to prevent activation of gene expression by a redox sensing gene circuit. </br><div class="center"> | Sulfite removes oxygen from solution allowing pyocyanin to be maintained in a reduced state. A potential of +0.5V generates oxidised pyocyanin and ferricyanide. Ferricyanide pushes the cell into an oxidising condition, allowing pyocyanin to remain oxidised and activate gene expression of a redox sensing gene circuit. A -0.3V potential generated reduced pyocyanin and ferrocyanide. Ferrocyanide pushes the cell into a reducing condition, allowing pyocyanin to remain reduced to prevent activation of gene expression by a redox sensing gene circuit. </br><div class="center"> | ||
| − | <img src="https://static.igem.org/mediawiki/2018/4/4c/T--Imperial_College--Naso3structure.png" alt="" width=" | + | <img src="https://static.igem.org/mediawiki/2018/4/4c/T--Imperial_College--Naso3structure.png" alt="" width="40%"; ></p2></div> |
</div> | </div> | ||
| Line 57: | Line 57: | ||
<div class="drop"> | <div class="drop"> | ||
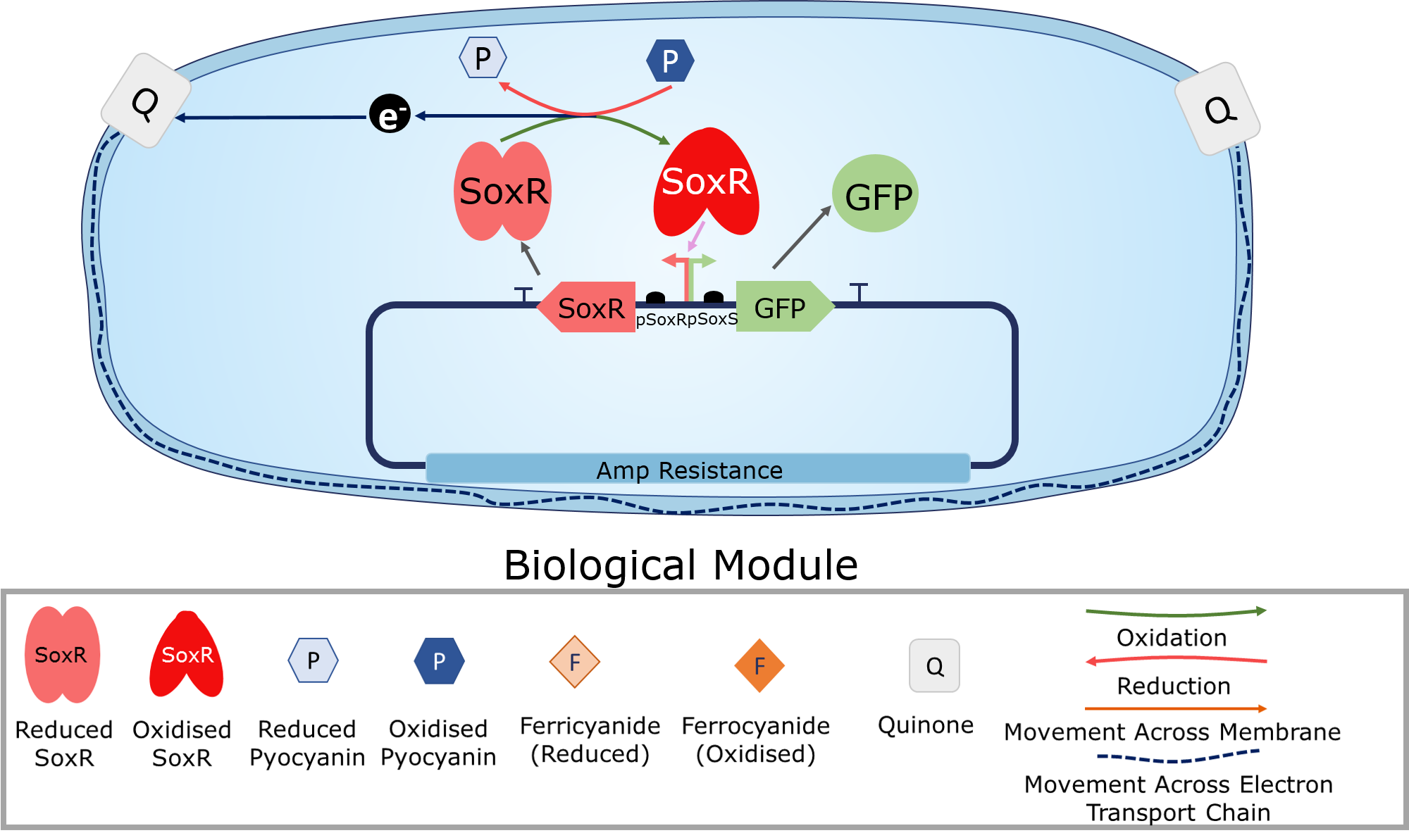
<p2>Oxidised redox molecules oxidise the transcription factor SoxR. This allows it to bind to and initiate transcription from the pSoxS promoter. This allows for the electronic induction of any gene placed downstream of this promoter.</p2> | <p2>Oxidised redox molecules oxidise the transcription factor SoxR. This allows it to bind to and initiate transcription from the pSoxS promoter. This allows for the electronic induction of any gene placed downstream of this promoter.</p2> | ||
| − | <img class="center" src="https://static.igem.org/mediawiki/2018/ | + | <img class="center" src="https://static.igem.org/mediawiki/2018/6/6d/T--Imperial_College--Biologicalmodulenew.png" alt="" width="50%";> |
</div> | </div> | ||
<button class="collapsible">Details</button> | <button class="collapsible">Details</button> | ||
| Line 80: | Line 80: | ||
<div class="drop"> | <div class="drop"> | ||
<p2>In ambient conditions or at -0.3V sulfite maintains pyocyanin in a reduced state whereas ferrocyanide is stable in its reduced form. The SoxR transcription factor therefore remains reduced, preventing induction of gene transcription from the pSoxS promoter.</p2> | <p2>In ambient conditions or at -0.3V sulfite maintains pyocyanin in a reduced state whereas ferrocyanide is stable in its reduced form. The SoxR transcription factor therefore remains reduced, preventing induction of gene transcription from the pSoxS promoter.</p2> | ||
| − | <img class="center" src="https://static.igem.org/mediawiki/2018/1/ | + | <img class="center" src="https://static.igem.org/mediawiki/2018/1/19/T--Imperial_College--Offstatesystemfinal.png" alt="" width="50%";> |
</div> | </div> | ||
<button class="collapsible">ON State</button> | <button class="collapsible">ON State</button> | ||
<div class="drop"> | <div class="drop"> | ||
<p2>An electrode pulse of +0.5V oxidises the redox molcules pyocyanin and ferrocyanide. Pyocyanin oxidises the SoxR transcription factor which in turn initiates transcription of any gene downstream of pSoxS. This electronic induction of gene expression is amplified by the oxidised ferricyanide which pulls electrons out of the respiratory transport chain.</p2> | <p2>An electrode pulse of +0.5V oxidises the redox molcules pyocyanin and ferrocyanide. Pyocyanin oxidises the SoxR transcription factor which in turn initiates transcription of any gene downstream of pSoxS. This electronic induction of gene expression is amplified by the oxidised ferricyanide which pulls electrons out of the respiratory transport chain.</p2> | ||
| − | <img class="center" src="https://static.igem.org/mediawiki/2018/ | + | <img class="center" src="https://static.igem.org/mediawiki/2018/9/96/T--Imperial_College--Onstatesystem.png" alt="" width="50%";> |
</div> | </div> | ||
<script> | <script> | ||
Latest revision as of 18:48, 17 October 2018
Design
Design Overview
Electrochemical Module Design

Potentiostat:
Electrode or Electrode Array:
Pyocyanin:

Ferrocyanide/Ferricyanide:


Sodium Sulfite:

Biological Module Design
SoxR:
pSoxS:
Quinone Pool:
Biological Module Mechanism:
When the Iron-Sulfur centres of SoxR are oxidised by oxidised pyocyanin produced by the electrochemical module, it activates transcription downstream of pSoxS. This allows for electrogenetic induction of any gene or gene circuit downstream of this promoter.PixCell Electrogenetic System Design















