| (11 intermediate revisions by 3 users not shown) | |||
| Line 42: | Line 42: | ||
<title>Collaborations</title> | <title>Collaborations</title> | ||
| − | + | <script> | |
| − | + | ||
| + | if(document.getElementById("home") != null) { | ||
| + | document.getElementById("homeheader").classList.add('current'); | ||
| + | } | ||
| + | if(document.getElementById("project") != null) { | ||
| + | document.getElementById("projecth").classList.add('current'); | ||
| + | } | ||
| + | if(document.getElementById("software") != null) { | ||
| + | document.getElementById("softwareh").classList.add('current'); | ||
| + | } | ||
| + | if(document.getElementById("parts") != null) { | ||
| + | document.getElementById("partsh").classList.add('current'); | ||
| + | } | ||
| + | if(document.getElementById("notebook") != null) { | ||
| + | document.getElementById("notebookh").classList.add('current'); | ||
| + | } | ||
| + | if(document.getElementById("human") != null) { | ||
| + | document.getElementById("humanh").classList.add('current'); | ||
| + | } | ||
| + | if(document.getElementById("people") != null) { | ||
| + | document.getElementById("peopleh").classList.add('current'); | ||
| + | } | ||
| + | </script> | ||
| + | |||
</head> | </head> | ||
| Line 49: | Line 72: | ||
| − | <body class="stretched"> | + | <body class="stretched sticky-responsive-pagemenu"> |
<!-- Document Wrapper | <!-- Document Wrapper | ||
| Line 92: | Line 115: | ||
<div class="swiper-container swiper-parent"> | <div class="swiper-container swiper-parent"> | ||
<div class="swiper-wrapper"> | <div class="swiper-wrapper"> | ||
| − | <div class="swiper-slide" style="background-image: url(' | + | <div class="swiper-slide" style="background-image: url('https://static.igem.org/mediawiki/2018/3/3f/T--Vilnius-Lithuania-OG--design.jpg');"> |
<div class="container clearfix"> | <div class="container clearfix"> | ||
<div class="slider-caption slider-caption-center"> | <div class="slider-caption slider-caption-center"> | ||
| − | <h2 data-caption-animate="fadeInUp | + | <h2 data-caption-animate="fadeInUp">Design</h2> |
<p class="d-none d-sm-block" data-caption-animate="fadeInUp" data-caption-delay="400"></p> | <p class="d-none d-sm-block" data-caption-animate="fadeInUp" data-caption-delay="400"></p> | ||
</div> | </div> | ||
| Line 109: | Line 132: | ||
</section> | </section> | ||
| + | |||
<!-- Page Sub Menu | <!-- Page Sub Menu | ||
============================================= --> | ============================================= --> | ||
| Line 119: | Line 143: | ||
<div class="menu-title">Explore <span>DESIGN</span></div> | <div class="menu-title">Explore <span>DESIGN</span></div> | ||
| − | + | <nav class="one-page-menu"> | |
<ul> | <ul> | ||
| − | <li><a href="#" style="font-size: 90%" | + | <li><a href="#" style="font-size: 90%" |
data-href="#section-introduction">Introduction</a></li> | data-href="#section-introduction">Introduction</a></li> | ||
| − | <li><a href="#" style="font-size: 90%"data-href="#section-library | + | <li><a href="#" style="font-size: 90%" data-href="#section-library">Library</a></li> |
| − | <li><a href="#" style="font-size: 90%"data-href="#section-encapsulation">Encapsulation</a></li> | + | <li><a href="#" style="font-size: 90%" data-href="#section-encapsulation">Encapsulation</a></li> |
| − | <li><a href="#" style="font-size: 90%"data-href="#section-production">Biomolecule Production</a></li> | + | <li><a href="#" style="font-size: 90%" data-href="#section-production">Biomolecule Production</a></li> |
| − | <li><a href="#" style="font-size: 90%"data-href="#section-conversion">Substrate Conversion</a></li> | + | <li><a href="#" style="font-size: 90%" data-href="#section-conversion">Substrate Conversion</a></li> |
| − | <li><a href="#" style="font-size: 90%"data-href="#section-recording">Recording</a></li> | + | <li><a href="#" style="font-size: 90%" data-href="#section-recording">Recording</a></li> |
| − | <li><a href="#" style="font-size: 90%"data-href="#section-reading">Reading</a></li> | + | <li><a href="#" style="font-size: 90%" data-href="#section-reading">Reading</a></li> |
</ul> | </ul> | ||
</nav> | </nav> | ||
| Line 143: | Line 167: | ||
============================================= --> | ============================================= --> | ||
| − | <section | + | |
| + | <section style="overflow:hidden; position:relative; background-color: #FFF;"> | ||
| + | |||
<div class="content-wrap"> | <div class="content-wrap"> | ||
| Line 158: | Line 184: | ||
<h2>Introduction</h2> | <h2>Introduction</h2> | ||
</div> | </div> | ||
| − | + | ||
<p>In this section you will find out what is <strong>Catalytic Activity Sequencing</strong> (CAT-Seq). Firstly, the CAT-Seq is introduced in brief to give a quick overview of how the system works and what are the different parts it consists of. Next, every step of the system is described and illustrated in detail. Finally, we show how we have applied this design in order to build the proof of concept of CAT-Seq.</p> | <p>In this section you will find out what is <strong>Catalytic Activity Sequencing</strong> (CAT-Seq). Firstly, the CAT-Seq is introduced in brief to give a quick overview of how the system works and what are the different parts it consists of. Next, every step of the system is described and illustrated in detail. Finally, we show how we have applied this design in order to build the proof of concept of CAT-Seq.</p> | ||
<p> </p> | <p> </p> | ||
| Line 169: | Line 195: | ||
<p>Currently, droplet microfluidics offer an efficient and high-throughput way to parallelise high number of reactions. As each molecule is encapsulated in a unique water droplet, various physically separated reactions can be carried out at the same time. Yet, what is <strong>missing</strong>, is a way to record the activity information in each droplet, so that after the droplets are collected together and broken, the sequence and function relationship is not lost. In other words, the main goal of CAT-Seq is to <strong>connect catalytic biomolecule’s phenotype to genotype in ultra-high throughput manner</strong>.</p> | <p>Currently, droplet microfluidics offer an efficient and high-throughput way to parallelise high number of reactions. As each molecule is encapsulated in a unique water droplet, various physically separated reactions can be carried out at the same time. Yet, what is <strong>missing</strong>, is a way to record the activity information in each droplet, so that after the droplets are collected together and broken, the sequence and function relationship is not lost. In other words, the main goal of CAT-Seq is to <strong>connect catalytic biomolecule’s phenotype to genotype in ultra-high throughput manner</strong>.</p> | ||
<p> </p> | <p> </p> | ||
| − | <p>During the theoretical design phase it became apparent that recording such information in mutant’s own DNA sequence from which it was synthesized would be the smartest choice. That is because <strong>DNA already contains half of the information</strong> we want to save - the sequence that codes the biomolecule. As we want to associate the sequence information with the information of catalytic activity, there did not seem to be a better object | + | <p>During the theoretical design phase it became apparent that recording such information in mutant’s own DNA sequence from which it was synthesized would be the smartest choice. That is because <strong>DNA already contains half of the information</strong> we want to save - the sequence that codes the biomolecule. As we want to associate the sequence information with the information of catalytic activity, there did not seem to be a better object than the <strong>DNA</strong>.</p> |
<div class="divider divider-center"><i class="icon-circle"></i></div> | <div class="divider divider-center"><i class="icon-circle"></i></div> | ||
| Line 175: | Line 201: | ||
<h4 class="excepth4">But how can we write such information into the DNA? Substrate Nucleotides!</h4> | <h4 class="excepth4">But how can we write such information into the DNA? Substrate Nucleotides!</h4> | ||
<p> </p> | <p> </p> | ||
| − | <p>While navigating the vast sea of scientific literature we have stumbled upon a fact that some polymerases do not incorporate nucleotides into DNA sequence <strong>if they are modified</strong>. We have then come up with an idea to <strong>attach the enzyme substrate | + | <p>While navigating the vast sea of scientific literature we have stumbled upon a fact that some polymerases do not incorporate nucleotides into DNA sequence <strong>if they are modified</strong>. We have then come up with an idea to <strong>attach the enzyme substrate to the nucleotides </strong>and term this compound<strong> “Substrate nucleotide”.</strong></p> |
| − | <img class="image_design" src=" | + | <img class="image_design" src="https://static.igem.org/mediawiki/2018/2/27/T--Vilnius-Lithuania-OG--nucleotide_1.jpeg"> |
| − | <p>Such modified nucleotides then act as <strong>a target for various | + | <p>Such modified nucleotides then act as <strong>a target for various catalytic biomolecules</strong> - they would first need to recognise it, bind to it and then chemically convert it, resulting in the removal of the substrate. We call this chemically converted nucleotide a “<strong>Product Nucleotide</strong>”.</p> |
| − | <p>The removal of the different substrates can be achieved by different types of catalytic biomolecules that catalyse a vast range of different chemical reactions. For example, all of the six major classes of enzymes can be used. To explore the prospects of recording the activities of different types of catalytic biomolecules, please head to the <strong>Substrate Design Prospects</strong> page!</p> | + | <p>The removal of the different substrates can be achieved by different types of catalytic biomolecules that catalyse a vast range of different chemical reactions. For example, all of the six major classes of enzymes can be used. To explore the prospects of recording the activities of different types of catalytic biomolecules, please head to the <a href="https://2018.igem.org/Team:Vilnius-Lithuania-OG/Nucleotide_Design"><strong>Substrate Design Prospects</strong></a> page!</p> |
<p> </p> | <p> </p> | ||
<p>The catalytic molecule acting on the<strong> substrate nucleotide</strong> is the main principle behind CAT-Seq. A substrate nucleotide <strong>cannot be incorporated</strong> by DNA polymerase. In contrast - <strong>Product Nucleotide can </strong>be incorporated.</p> | <p>The catalytic molecule acting on the<strong> substrate nucleotide</strong> is the main principle behind CAT-Seq. A substrate nucleotide <strong>cannot be incorporated</strong> by DNA polymerase. In contrast - <strong>Product Nucleotide can </strong>be incorporated.</p> | ||
| Line 200: | Line 226: | ||
<h3>Design Overview</h3> | <h3>Design Overview</h3> | ||
| + | <img class="image_design" src="https://static.igem.org/mediawiki/2018/9/9f/T--Vilnius-Lithuania-OG--catdesignrevieworbsmolecule.jpeg"> | ||
<h3>Design description</h3> | <h3>Design description</h3> | ||
| Line 210: | Line 237: | ||
<p class="didesnist"><strong>General description</strong></p> | <p class="didesnist"><strong>General description</strong></p> | ||
| − | <img class="image_design_side" style="width: 60%;float: right;" src=" | + | <img class="image_design_side" style="width: 60%;float: right;" src="https://static.igem.org/mediawiki/2018/b/be/T--Vilnius-Lithuania-OG--circulization1.jpeg"> |
<p>A library of is prepared <strong>(1)</strong> for a specific or group of catalytic biomolecules - <strong>enzymes</strong>, <strong>catalytic RNAs</strong> or <strong>catalytic DNAs</strong>. For example, such library may consist of billions of randomly mutated enzyme sequences, a few computationally derived enzymes or a <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="Metagenomics is the study of genetic material recovered directly from environmental samples">metagenomic</a> enzyme library.</p> | <p>A library of is prepared <strong>(1)</strong> for a specific or group of catalytic biomolecules - <strong>enzymes</strong>, <strong>catalytic RNAs</strong> or <strong>catalytic DNAs</strong>. For example, such library may consist of billions of randomly mutated enzyme sequences, a few computationally derived enzymes or a <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="Metagenomics is the study of genetic material recovered directly from environmental samples">metagenomic</a> enzyme library.</p> | ||
| Line 216: | Line 243: | ||
<p>After the regulatory parts are added the fragments must then be <strong>circularized (3)</strong>. DNA fragments are circularized for two main reasons:</p> | <p>After the regulatory parts are added the fragments must then be <strong>circularized (3)</strong>. DNA fragments are circularized for two main reasons:</p> | ||
| − | <p class="listin">1. First of all, in order to amplify the DNA inside microfluidic droplets an <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="Isothermal amplification reactions covers multiple methods which utilize the amplification reaction at a single-temperature, usually are extremely fast and do not require thermocyclers">isothermal amplification reaction</a> must be used. That is because droplets become highly unstable and can break easily when temperature is quickly | + | <p class="listin">1. First of all, in order to amplify the DNA inside microfluidic droplets an <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="Isothermal amplification reactions covers multiple methods which utilize the amplification reaction at a single-temperature, usually are extremely fast and do not require thermocyclers">isothermal amplification reaction</a> must be used. That is because droplets become highly unstable and can break easily when the temperature is quickly altered - just like during the popular polymerase chain reaction (PCR). If droplets would break during the initial stages of amplification, all of the information from different droplets would get mixed in the tube and the knowledge about which activity belongs to which sequence would be lost, as it was not yet recorded by the means of amplification (before the amplification, the activity measurement information is in form of modified substrate nucleotides).<br /> That is why CAT-Seq uses a multiple displacement amplification (MDA), which is a non-thermocycling (isothermal) based DNA amplification technique. MDA was chosen because of its ability to rapidly amplify single templates of DNA into long double-stranded molecules which contain multiple repeats of the starting template. That said, one of the core requirements of MDA reaction is that starting DNA template <strong>must be circular</strong>.</p> |
<p class="listin">2. Secondly, as seen in the later steps of CAT-Seq, in order to efficiently express a catalytic biomolecule in droplets, <strong>Rolling Cycle Transcription</strong> (described in catalytic biomolecule production section) is used, which immensely increases mRNA synthesis yield from a single DNA template. Such technique also <strong>requires the DNA to be circular</strong>, as an RNA polymerase travels around it in circles producing mRNA.</p> | <p class="listin">2. Secondly, as seen in the later steps of CAT-Seq, in order to efficiently express a catalytic biomolecule in droplets, <strong>Rolling Cycle Transcription</strong> (described in catalytic biomolecule production section) is used, which immensely increases mRNA synthesis yield from a single DNA template. Such technique also <strong>requires the DNA to be circular</strong>, as an RNA polymerase travels around it in circles producing mRNA.</p> | ||
| Line 227: | Line 254: | ||
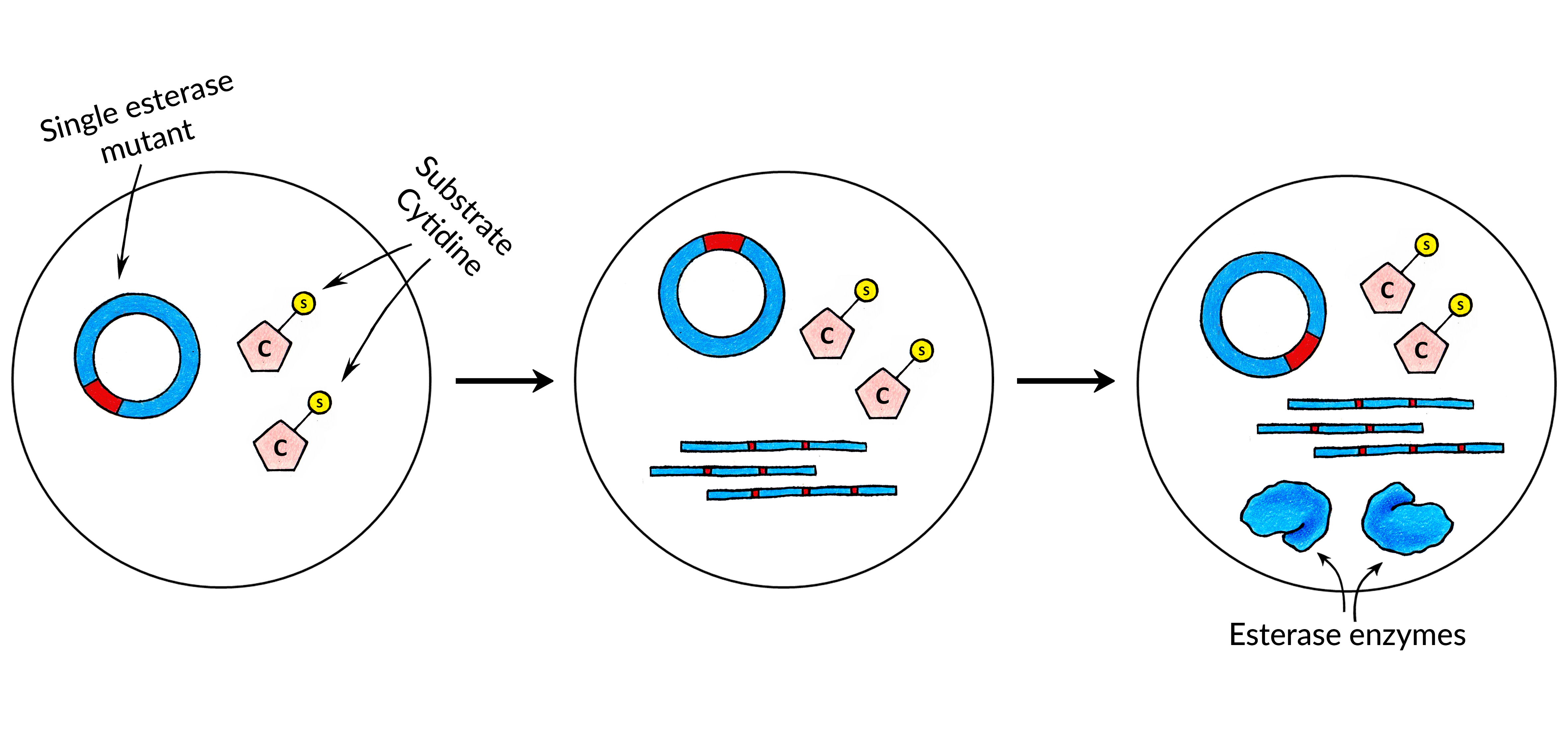
<p><strong>Obtaining the first catalytic biomolecule</strong></p> | <p><strong>Obtaining the first catalytic biomolecule</strong></p> | ||
<p>In order to find a correct combination of Substrate Nucleotide and the Catalytic Biomolecule that can catalyze the removal of the substrate, we have decided to look for a hydrolase. The is because a direct removal of the substrate from nucleotide seemed like the most timewise efficient choice.</p> | <p>In order to find a correct combination of Substrate Nucleotide and the Catalytic Biomolecule that can catalyze the removal of the substrate, we have decided to look for a hydrolase. The is because a direct removal of the substrate from nucleotide seemed like the most timewise efficient choice.</p> | ||
| − | <p> First, we synthesized a cytidine which had a benzoyl substrate attached to it: <strong>N4-benzoyl-2'-deoxycytidine triphosphate</strong>. Instructions | + | <p> First, we synthesized a cytidine which had a benzoyl substrate attached to it: <strong>N4-benzoyl-2'-deoxycytidine triphosphate</strong>. Instructions on how such substrate nucleotide can be synthesized can be found here.</p> |
<p>Then, an <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis">esterase</a> library was screened manually using microwell plates in order to find the hydrolase enzyme which can catalyse the removal of the substrate. Once such enzyme was found and its sequence was determined. We have then used this pair of <strong>esterase enzyme</strong> and <strong>substrate cytidine</strong> to build the first version of CAT-Seq.</p> | <p>Then, an <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis">esterase</a> library was screened manually using microwell plates in order to find the hydrolase enzyme which can catalyse the removal of the substrate. Once such enzyme was found and its sequence was determined. We have then used this pair of <strong>esterase enzyme</strong> and <strong>substrate cytidine</strong> to build the first version of CAT-Seq.</p> | ||
| − | <img class="image_design" style="margin-bottom: 3%;" src=" | + | <img class="image_design" style="margin-bottom: 3%;" src="https://static.igem.org/mediawiki/2018/e/e9/T--Vilnius-Lithuania-OG--substrate.jpeg"> |
| − | <p>Next, we have performed an in-silico modelling of our Esterase enzyme. The goal of the in-silico analysis was to create the Esterase mutants which would have | + | <p>Next, we have performed an in-silico modelling of our Esterase enzyme. The goal of the in-silico analysis was to create the Esterase mutants which would have different catalytic activities. Once such mutants were constructed, their relative catalytic activities were measured again using the same standard methods we have used to find the initial esterase enzyme. This was done in order to construct a small Esterase mutant library which would have precisely-defined catalytic activities by already established low-throughput measuring methods. Then, with CAT-Seq, we can also measure the activities of those mutants in high-throughput, which can then be <strong>compared to our previous measurements</strong> to confirm that CAT-Seq system is working as intended and can precisely discriminate different mutant activities.</p> |
| − | <p> After acquiring the esterase enzyme and its mutants, <strong>regulatory sequences </strong>- RNA polymerase promoter and ribosome binding site - were added to each of the library | + | <p> After acquiring the esterase enzyme and its mutants, <strong>regulatory sequences </strong>- RNA polymerase promoter and ribosome binding site - were added to each of the library members. Then, the fragments were <strong>circularized </strong>for the MDA and RCT reactions.</p> |
<p><strong>After acquiring the initial esterase for building CAT-Seq, why were the mutations added manually and not randomly?</strong></p> | <p><strong>After acquiring the initial esterase for building CAT-Seq, why were the mutations added manually and not randomly?</strong></p> | ||
<p>The goal of the in-silico modelling of the esterase was to determine mutants that would have different catalytic activities. If we had a randomized library of esterase mutants, it would have been difficult to precisely tune CAT-Seq parts as we would not be sure what output of the system we should expect. In contrast, having mutants with precisely measured catalytic activities beforehand using well-established and precise low-throughput methods enables us to recognise how well our system is performing. </p> | <p>The goal of the in-silico modelling of the esterase was to determine mutants that would have different catalytic activities. If we had a randomized library of esterase mutants, it would have been difficult to precisely tune CAT-Seq parts as we would not be sure what output of the system we should expect. In contrast, having mutants with precisely measured catalytic activities beforehand using well-established and precise low-throughput methods enables us to recognise how well our system is performing. </p> | ||
| Line 250: | Line 277: | ||
<p>Using a microfluidic device chip whole DNA library can be encapsulated into the droplets, together with specific reaction reagents. Water phase flows through the microfluidic device channels with the help of pressure created by special pumps.</p> | <p>Using a microfluidic device chip whole DNA library can be encapsulated into the droplets, together with specific reaction reagents. Water phase flows through the microfluidic device channels with the help of pressure created by special pumps.</p> | ||
| − | <img class="image_design_side" style="width: 50%; float: right" src=" | + | <img class="image_design_side" style="width: 50%; float: right" src="https://static.igem.org/mediawiki/2018/5/53/T--Vilnius-Lithuania-OG--droplet_formation.jpeg"> |
| Line 259: | Line 286: | ||
<p class="listin"><strong>3. Substrate Nucleotides</strong> - the target for catalytic biomolecule. It can be attached to any of the four <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="dCTP, dGTP, dATP, dTTP">dNTPs</a>. All four different nucleotides can be used in order to record the activity information using 4 different substrates.<br />The goal of the catalytic biomolecule is to recognise the substrate and catalyze a particular chemical conversion which depends on the substrate and biomolecules type. In many cases such conversion helps the substrate to detach from the nucleotide. Such detachment can be achieved by the first catalyzed reaction or subsequent spontaneous reactions that follow the initial one.</p> | <p class="listin"><strong>3. Substrate Nucleotides</strong> - the target for catalytic biomolecule. It can be attached to any of the four <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="dCTP, dGTP, dATP, dTTP">dNTPs</a>. All four different nucleotides can be used in order to record the activity information using 4 different substrates.<br />The goal of the catalytic biomolecule is to recognise the substrate and catalyze a particular chemical conversion which depends on the substrate and biomolecules type. In many cases such conversion helps the substrate to detach from the nucleotide. Such detachment can be achieved by the first catalyzed reaction or subsequent spontaneous reactions that follow the initial one.</p> | ||
| − | <img class="image_design" style="margin-bottom: 3%; width: 80%; margin: auto; display: block;" src=" | + | <img class="image_design" style="margin-bottom: 3%; width: 80%; margin: auto; display: block;" src="https://static.igem.org/mediawiki/2018/d/dc/T--Vilnius-Lithuania-OG--before_after.jpeg"> |
<p>Please see the Methods and Protocols section for a more detailed description of microfluidics techniques.</p> | <p>Please see the Methods and Protocols section for a more detailed description of microfluidics techniques.</p> | ||
| Line 269: | Line 296: | ||
<p class="listin"><strong>3. Substrate Nucleotides</strong> (N4-benzoyl-2'-deoxycytidine triphosphates) as the targets for the esterase enzyme. </p> | <p class="listin"><strong>3. Substrate Nucleotides</strong> (N4-benzoyl-2'-deoxycytidine triphosphates) as the targets for the esterase enzyme. </p> | ||
| − | <p>How such encapsulation looks can be seen in the video below:</p> | + | <p>How such encapsulation looks can be seen in the video below (Video slowed down 2000x):</p> |
| + | <img style="margin-bottom: 18%; width: 100%; margin: auto; display: block;" src="https://static.igem.org/mediawiki/2018/1/1f/T--Vilnius-Lithuania-OG--encapsulation.gif"> | ||
| − | + | <p style="margin-top: 5%;">After the encapsulation each droplet on average contains single copy of a mutant DNA sequence from the Esterase library. Also every droplet contains the same amount of IVTT reagents and Substrate Nucleotides. We call these droplets the <strong>Substrate Droplets</strong>.</p> | |
| − | <p>After the encapsulation each droplet on average contains single copy of a mutant DNA sequence from the Esterase library. Also every droplet contains the same amount of IVTT reagents and Substrate Nucleotides. We call these droplets the <strong>Substrate Droplets</strong>.</p> | + | |
<div class="toggle toggle-bg"> | <div class="toggle toggle-bg"> | ||
<div class="togglet"><i class="toggle-closed icon-ok-circle"></i><i class="toggle-open icon-remove-circle"></i><strong>After encapsulation, does every droplet contain only a single DNA fragment from the library?</strong></div> | <div class="togglet"><i class="toggle-closed icon-ok-circle"></i><i class="toggle-open icon-remove-circle"></i><strong>After encapsulation, does every droplet contain only a single DNA fragment from the library?</strong></div> | ||
| Line 279: | Line 306: | ||
<p>In order to ensure the fidelity of the data collected using CAT-Seq only every 10th droplet houses a DNA template after droplet generation. If the concentration of DNA molecules in the starting encapsulation mix is too high, the likelihood of encapsulating multiple mutant templates per droplet increases drastically. Such events disrupt the data acquired by CAT-Seq or any droplet microfluidics technique. Encapsulation events can be precisely described by Poisson distribution:</p> | <p>In order to ensure the fidelity of the data collected using CAT-Seq only every 10th droplet houses a DNA template after droplet generation. If the concentration of DNA molecules in the starting encapsulation mix is too high, the likelihood of encapsulating multiple mutant templates per droplet increases drastically. Such events disrupt the data acquired by CAT-Seq or any droplet microfluidics technique. Encapsulation events can be precisely described by Poisson distribution:</p> | ||
| − | <img class="image_design" style="padding-bottom: 4%;; width: 30%; margin: auto; display: block;" src=" | + | <img class="image_design" style="padding-bottom: 4%;; width: 30%; margin: auto; display: block;" src="https://static.igem.org/mediawiki/2018/3/37/T--Vilnius-Lithuania-OG--poisson_formula.png"> |
| − | <p>P(X = k) is the probability for single droplet to house k DNA molecules. λ shows the average number of DNA molecules per droplet. For example, if you want to have a single template per droplets, you might choose λ to be 1. The Poisson distribution predicts, that 36.7% of droplets will have one molecule. However, it also predicts that 18.4% of droplets will house 2 molecules and 6.1% - 3 molecules. Following this notation, In order to avoid artificial data, caused by multiple DNA templates in one droplet, the concentration of DNA template was kept at 1 per 10 droplets (λ = 0.1). As seen from the graph, 90.4% of droplets will be empty and 9% will have 1 template. On the other hand, 0.4%. of droplets will house 2 DNA molecules, meaning that droplets with 2 DNA templates are generated rarely</p> | + | <p>P(X = k) is the probability for the single droplet to house k DNA molecules. λ shows the average number of DNA molecules per droplet. For example, if you want to have a single template per droplets, you might choose λ to be 1. The Poisson distribution predicts, that 36.7% of droplets will have one molecule. However, it also predicts that 18.4% of droplets will house 2 molecules and 6.1% - 3 molecules. Following this notation, In order to avoid artificial data, caused by multiple DNA templates in one droplet, the concentration of the DNA template was kept at 1 per 10 droplets (λ = 0.1). As seen from the graph, 90.4% of droplets will be empty and 9% will have 1 template. On the other hand, 0.4%. of droplets will house 2 DNA molecules, meaning that droplets with 2 DNA templates are generated rarely</p> |
| − | <img class="image_design" style="margin-bottom: 3%; width: 100%; margin: auto; display: block;" src=" | + | <img class="image_design" style="margin-bottom: 3%; width: 100%; margin: auto; display: block;" src="https://static.igem.org/mediawiki/2018/8/89/T--Vilnius-Lithuania-OG--graph.png"> |
</div> | </div> | ||
</div> | </div> | ||
| Line 298: | Line 325: | ||
<p class="listin">1. <strong> Nucleic Acids</strong>: encapsulating DNA or RNA molecules encoding the catalytic biomolecule. After the encapsulation the droplets can be incubated in order to produce the biomolecule. If the catalytic biomolecule is an enzyme, <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="the production of protein using biological machinery in a cell-free system, that is, without the use of living cells">in-vitro protein synthesis</a> (<b>A</b>) kit can be used in order to produce it. Otherwise, if it is a <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="RNA molecule capable of catalyzing a chemical reaction">Ribozyme</a> (<b>B</b>), it can be produced using <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="the production of RNA using biological machinery in a cell-free system, that is, without the use of living cells">in-vitro transcription</a> reagents. Also, there are cases where the catalytic biomolecule is a <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="DNA molecule capable of catalyzing a chemical reaction">Deoxyribozyme</a> (<b>C</b>). Then, all that is required is to encapsulate the DNA library!</p> | <p class="listin">1. <strong> Nucleic Acids</strong>: encapsulating DNA or RNA molecules encoding the catalytic biomolecule. After the encapsulation the droplets can be incubated in order to produce the biomolecule. If the catalytic biomolecule is an enzyme, <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="the production of protein using biological machinery in a cell-free system, that is, without the use of living cells">in-vitro protein synthesis</a> (<b>A</b>) kit can be used in order to produce it. Otherwise, if it is a <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="RNA molecule capable of catalyzing a chemical reaction">Ribozyme</a> (<b>B</b>), it can be produced using <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="the production of RNA using biological machinery in a cell-free system, that is, without the use of living cells">in-vitro transcription</a> reagents. Also, there are cases where the catalytic biomolecule is a <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="DNA molecule capable of catalyzing a chemical reaction">Deoxyribozyme</a> (<b>C</b>). Then, all that is required is to encapsulate the DNA library!</p> | ||
| − | <img class="image_design" style=" margin-top: -3%; margin-bottom: 2%; display: block;" src=" | + | <img class="image_design" style=" margin-top: -3%; margin-bottom: 2%; display: block;" src="https://static.igem.org/mediawiki/2018/c/c0/T--Vilnius-Lithuania-OG--catalytic_molecules.jpeg"> |
<p> During one of the collaborative meetings with experts from microfluidic technology field in Life Sciences Center located in Vilnius, we have received information that synthesizing biomolecules from a single DNA template is an <strong>extremely inefficient process</strong>. That is because when there is only a single template of DNA, the mRNA synthesis yield is extremely low and there is <strong>not enough mRNA </strong>to make an enzyme.</p> | <p> During one of the collaborative meetings with experts from microfluidic technology field in Life Sciences Center located in Vilnius, we have received information that synthesizing biomolecules from a single DNA template is an <strong>extremely inefficient process</strong>. That is because when there is only a single template of DNA, the mRNA synthesis yield is extremely low and there is <strong>not enough mRNA </strong>to make an enzyme.</p> | ||
| − | <img class="image_design" style="margin-bottom: 3%;display: block;" src=" | + | <img class="image_design" style="margin-bottom: 3%;display: block;" src="https://static.igem.org/mediawiki/2018/9/9f/T--Vilnius-Lithuania-OG--no_protein.jpeg"> |
| − | <p>After doing in depth literature research to try | + | <p>After doing in-depth literature research to try to solve this problem, we have found out that around 1990s people immensely increased short microRNA synthesis yields by circularizing DNA templates. As RNA polymerase goes around the DNA in circles it produces long strands of RNA molecules. Within those long molecules were the repeating small units of microRNA. They coined this method <strong>Rolling Circle Transcription</strong> (RCT). </p> |
| − | <img class="image_design" style="margin-bottom: 3%;display: block;" src=" | + | <img class="image_design" style="margin-bottom: 3%;display: block;" src="https://static.igem.org/mediawiki/2018/a/a1/T--Vilnius-Lithuania-OG--rct.jpeg"> |
<p>Following this discovery, we have figured out we can apply <strong>RCT </strong>in order to fix the current fundamental issues with biomolecule expression from single DNA templates in the field of droplet microtechnologies. As we are already circularizing DNA molecules in order to perform the MDA reaction in later steps, all that is left to do is to <strong>remove the transcription terminator</strong> from the catalytic biomolecule library members. Such removal of the terminator highly increases the mRNA concentration from a single template. Therefore, enzymes can then be <strong>efficiently synthesized</strong> using a <strong>single template of DNA</strong>.</p> | <p>Following this discovery, we have figured out we can apply <strong>RCT </strong>in order to fix the current fundamental issues with biomolecule expression from single DNA templates in the field of droplet microtechnologies. As we are already circularizing DNA molecules in order to perform the MDA reaction in later steps, all that is left to do is to <strong>remove the transcription terminator</strong> from the catalytic biomolecule library members. Such removal of the terminator highly increases the mRNA concentration from a single template. Therefore, enzymes can then be <strong>efficiently synthesized</strong> using a <strong>single template of DNA</strong>.</p> | ||
| − | <img class="image_design" style="margin-top: -2%;display: block;" src=" | + | <img class="image_design" style="margin-top: -2%;display: block;" src="https://static.igem.org/mediawiki/2018/3/3b/T--Vilnius-Lithuania-OG--rna_synthesis.jpeg"> |
| − | <p class="listin">2. <strong> Cells</strong>: catalytic biomolecules can also be provided into droplets in the form of cells, which have the DNA library <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="transformation is the genetic alteration of a cell resulting from the direct uptake and incorporation of genetic material from its surroundings through the cell membrane"><strong>transformed</strong></a> into them (one library variant per cell on average). In turn, the cells may produce the protein of interest. After cells are encapsulated into droplets, cell specific reagents can lyse the cells. After the cell lysis, enzyme of interest is released into the droplet together with DNA molecules that encoded it.</p> | + | <p class="listin">2. <strong> Cells</strong>: catalytic biomolecules can also be provided into droplets in the form of cells, which have the DNA library <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="transformation is the genetic alteration of a cell resulting from the direct uptake and incorporation of genetic material from its surroundings through the cell membrane"><strong>transformed</strong></a> into them (one library variant per cell on average). In turn, the cells may produce the protein of interest. After cells are encapsulated into droplets, cell-specific reagents can lyse the cells. After the cell lysis, an enzyme of interest is released into the droplet together with DNA molecules that encoded it.</p> |
| − | <img class="image_design" style="margin-top: -2%;display: block; margin-bottom: -4%" src=" | + | <img class="image_design" style="margin-top: -2%;display: block; margin-bottom: -4%" src="https://static.igem.org/mediawiki/2018/7/7f/T--Vilnius-Lithuania-OG--cells.jpeg"> |
| Line 327: | Line 354: | ||
| − | <img class="image_design" style="margin-top: -3%;display: block;" src=" | + | <img class="image_design" style="margin-top: -3%;display: block;" src="https://static.igem.org/mediawiki/2018/1/11/T--Vilnius-Lithuania-OG--long_rna.jpeg"> |
| Line 340: | Line 367: | ||
| − | <img class="image_design" style="margin-top: 0%; margin-bottom: 3%;display: block;" src=" | + | <img class="image_design" style="margin-top: 0%; margin-bottom: 3%;display: block;" src="https://static.igem.org/mediawiki/2018/3/34/T--Vilnius-Lithuania-OG--different_activity.jpeg"> |
| Line 346: | Line 373: | ||
<p>In each droplet esterase mutants are synthesized. Depending on their activities, they will remove different amount of substrates from the Substrate Cytidines in different droplets.</p> | <p>In each droplet esterase mutants are synthesized. Depending on their activities, they will remove different amount of substrates from the Substrate Cytidines in different droplets.</p> | ||
| − | <img class="image_design" style="margin-top: 0%; margin-bottom: 3%;display: block;" src=" | + | <img class="image_design" style="margin-top: 0%; margin-bottom: 3%;display: block;" src="https://static.igem.org/mediawiki/2018/c/c3/T--Vilnius-Lithuania-OG--Esterase_mutants.jpeg"> |
<div class="toggle toggle-bg"> | <div class="toggle toggle-bg"> | ||
| − | <div class="togglet"><i class="toggle-closed icon-ok-circle"></i><i class="toggle-open icon-remove-circle"></i><div style="; font-size: 90%; margin-right: 5%; ">Does the design choice of removing substrate | + | <div class="togglet"><i class="toggle-closed icon-ok-circle"></i><i class="toggle-open icon-remove-circle"></i><div style="; font-size: 90%; margin-right: 5%; ">Does the design choice of removing substrate limit the different class of biomolecules we can analyse?</div></div> |
<div class="togglec"> | <div class="togglec"> | ||
| − | <p>Not at all! CAT-Seq allows to screen biomolecules catalyzing numerous distinct reactions. Please head to the Substrate design page for more information and examples!</p> | + | <p>Not at all! CAT-Seq allows to screen biomolecules catalyzing numerous of distinct reactions. Please head to the <a href="https://2018.igem.org/Team:Vilnius-Lithuania-OG/Nucleotide_Design">Substrate Nucleotide design page</a> for more information and examples!</p> |
</div> | </div> | ||
</div> | </div> | ||
| Line 371: | Line 398: | ||
| − | <img class="image_design" style="margin: auto; padding-bottom: 4%; width: 70%; display: block;" src=" | + | <img class="image_design" style="margin: auto; padding-bottom: 4%; width: 70%; display: block;" src="https://static.igem.org/mediawiki/2018/8/89/T--Vilnius-Lithuania-OG--droplets_merge.jpeg"> |
| − | <p>Another important thing to consider is creating a reference point which would help to precisely determine the | + | <p>Another important thing to consider is creating a reference point which would help to precisely determine the activities of different catalytic biomolecules. For CAT-Seq, such reference point is nucleotides with reference modification. This modification can be any small modification which still allows a DNA polymerase to incorporate it during the amplification. We call them the <strong>Reference Nucleotides. </strong></p> |
| − | <p>For example, If we would try to amplify DNA template in each droplet that contains different amounts of nucleotides that can be incorporated, we would get a sequencing <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="Bias is disproportionate weight in | + | <p>For example, If we would try to amplify DNA template in each droplet that contains different amounts of nucleotides that can be incorporated, we would get a sequencing <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="Bias is disproportionate weight in favour of or against one thing compared with another">bias</a> that would be more favourable for biomolecules that are highly active.</p> |
| − | <img class="image_design" style="margin-top: -3%; margin-bottom: 2%; display: block;" src=" | + | <img class="image_design" style="margin-top: -3%; margin-bottom: 2%; display: block;" src="https://static.igem.org/mediawiki/2018/5/53/T--Vilnius-Lithuania-OG--dna_amplification.jpeg"> |
| − | <p>That is because biomolecules with higher activity would produce more nucleotides for polymerase and the amplified DNA amount would be much bigger | + | <p>That is because biomolecules with higher activity would produce more nucleotides for polymerase and the amplified DNA amount would be much bigger than compared to lower activity mutants which would produce less. Reference nucleotides can easily remove this bias - after merging the Substrate Droplet with Amplification droplets that contain Reference Nucleotides, they allow the synthesis of a similar amount of amplified DNA in each droplet.</p> |
| − | <img class="image_design" style="margin-top: -3%; margin-bottom: 2%; display: block;" src=" | + | <img class="image_design" style="margin-top: -3%; margin-bottom: 2%; display: block;" src="https://static.igem.org/mediawiki/2018/a/ae/T--Vilnius-Lithuania-OG--amplification_2.jpeg"> |
| Line 394: | Line 421: | ||
<p style="margin-bottom: 0%;">Going back, we now have a population of <strong>substrate droplets</strong> that have a defined amount <strong>Product Nucleotides</strong> (the chemically converted substrate nucleotides). Those must then be merged with <strong>Amplification Droplets</strong>, wherein every droplet contains the same amount of amplification reagents and <strong>Reference Nucleotides</strong>. After the droplet merging, each droplet now contains a <strong>ratio of Product nucleotides to Reference Nucleotides</strong>. If the catalytic biomolecule was highly active, the ratio of Product Nucleotides to Reference Nucleotides will be high. In contrast, if the biomolecule activity was low, the ratio will also be low.</p> | <p style="margin-bottom: 0%;">Going back, we now have a population of <strong>substrate droplets</strong> that have a defined amount <strong>Product Nucleotides</strong> (the chemically converted substrate nucleotides). Those must then be merged with <strong>Amplification Droplets</strong>, wherein every droplet contains the same amount of amplification reagents and <strong>Reference Nucleotides</strong>. After the droplet merging, each droplet now contains a <strong>ratio of Product nucleotides to Reference Nucleotides</strong>. If the catalytic biomolecule was highly active, the ratio of Product Nucleotides to Reference Nucleotides will be high. In contrast, if the biomolecule activity was low, the ratio will also be low.</p> | ||
| − | <img class="image_design" style="width: 70%; display: block; margin: auto; padding-top: -5%;" src=" | + | <img class="image_design" style="width: 70%; display: block; margin: auto; padding-top: -5%;" src="https://static.igem.org/mediawiki/2018/4/42/T--Vilnius-Lithuania-OG--ratio.jpeg"> |
<p style="margin-bottom">These specific nucleotide ratios in droplets can then be <strong>recorded into DNA, by the means of DNA amplification</strong>. As DNA is amplified in each droplet, specific ratio of nucleotides is incorporated into the copy of initial template. Such amplification results in molecules that both encode the <strong>sequence of a specific biomolecule </strong>and information about that particular biomolecules’ <strong>catalytic activity</strong>.</p> | <p style="margin-bottom">These specific nucleotide ratios in droplets can then be <strong>recorded into DNA, by the means of DNA amplification</strong>. As DNA is amplified in each droplet, specific ratio of nucleotides is incorporated into the copy of initial template. Such amplification results in molecules that both encode the <strong>sequence of a specific biomolecule </strong>and information about that particular biomolecules’ <strong>catalytic activity</strong>.</p> | ||
| − | <img class="image_design" style="; display: block; margin: auto; " src=" | + | <img class="image_design" style="; display: block; margin: auto; " src="https://static.igem.org/mediawiki/2018/b/b1/T--Vilnius-Lithuania-OG--ratio_2.jpeg"> |
<p class="didesnist"><strong>Proof of concept</strong></p> | <p class="didesnist"><strong>Proof of concept</strong></p> | ||
<p>We now have a population of droplets, wherein each droplet contains a <strong>specific amount of Product Nucleotides</strong> or 2'-deoxycytidine triphosphates.</p> | <p>We now have a population of droplets, wherein each droplet contains a <strong>specific amount of Product Nucleotides</strong> or 2'-deoxycytidine triphosphates.</p> | ||
| − | <p>As already mentioned earlier, we have chosen to use MDA reaction in order to amplify the DNA isothermally. Isothermal amplification allows us to amplify the DNA without disrupting the stability of the droplets. Also, for the Reference Nucleotides we have decided to use Methylated Cytidines (2'-deoxy-5-methylcytidine 5'-triphosphates). These Reference Nucleotides can be readily incorporated by multiple polymerases, including Phi29 which we use for our MDA reaction.</p> | + | <p>As already mentioned earlier, we have chosen to use MDA reaction in order to amplify the DNA isothermally. Isothermal amplification allows us to amplify the DNA without disrupting the stability of the droplets. Also, for the Reference Nucleotides, we have decided to use Methylated Cytidines (2'-deoxy-5-methylcytidine 5'-triphosphates). These Reference Nucleotides can be readily incorporated by multiple polymerases, including Phi29 which we use for our MDA reaction.</p> |
<p>Next, we need to merge our Substrate Droplets with the Amplification Droplets containing the MDA reaction mix. Droplet merging is not an easy task to achieve, as efficient merging requires precise tuning of multiple parameters, such as 4 different droplet and oil flow rates and the strength of the electrical field that helps the droplets to merge. Searching for these parameters experimentally can often be like searching for a needle in a haystack. For this reason, before starting to do droplet merging experiments in the laboratory, we have written a thorough mathematical model which describes the droplet merging in detail and has the ability to predict the correct experimental parameters. We have successfully determined those <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="Water and oil phase flow rates, different droplet volume ratios, the strength of the electrical field and other">parameters</a> and showed experimentally, that they were indeed well-performing conditions for the droplet merging.</p> | <p>Next, we need to merge our Substrate Droplets with the Amplification Droplets containing the MDA reaction mix. Droplet merging is not an easy task to achieve, as efficient merging requires precise tuning of multiple parameters, such as 4 different droplet and oil flow rates and the strength of the electrical field that helps the droplets to merge. Searching for these parameters experimentally can often be like searching for a needle in a haystack. For this reason, before starting to do droplet merging experiments in the laboratory, we have written a thorough mathematical model which describes the droplet merging in detail and has the ability to predict the correct experimental parameters. We have successfully determined those <a href="javascript:;" data-toggle="tooltip" title="" data-original-title="Water and oil phase flow rates, different droplet volume ratios, the strength of the electrical field and other">parameters</a> and showed experimentally, that they were indeed well-performing conditions for the droplet merging.</p> | ||
<p>Substrate Droplets could then be merged with Amplification Droplets containing MDA mix and Reference Nucleotides.</p> | <p>Substrate Droplets could then be merged with Amplification Droplets containing MDA mix and Reference Nucleotides.</p> | ||
| − | <p>Each merged droplet now contains a specific ratio of Product Cytidines to Methylated Cytidines. Such information can then | + | <p>Each merged droplet now contains a specific ratio of Product Cytidines to Methylated Cytidines. Such information can then be recorded by initiating MDA reaction. After the reaction, the amplified DNA molecules now have the specific ratio of nucleotides, wherein the ratio of those nucleotides depend on the specific esterase mutant activity.</p> |
| − | <img class="image_design" style="; display: block; margin-bottom: 2%; " src=" | + | <img class="image_design" style="; display: block; margin-bottom: 2%; " src="https://static.igem.org/mediawiki/2018/a/ac/T--Vilnius-Lithuania-OG--ratio_3.jpeg"> |
<section id="section-reading" class="page-section"> | <section id="section-reading" class="page-section"> | ||
| Line 422: | Line 449: | ||
<p class="didesnist"><strong>General description</strong></p> | <p class="didesnist"><strong>General description</strong></p> | ||
| − | <p>The last step of the CAT-Seq is the retrieval of recorded activity information from the amplified DNA. In theory, this may achieved by any sequencing method that can incorporate a DNA modification recognition step. Yet, CAT-Seq uses <strong>Nanopore Sequencing</strong> for several reasons:</p> | + | <p>The last step of the CAT-Seq is the retrieval of recorded activity information from the amplified DNA. In theory, this may be achieved by any sequencing method that can incorporate a DNA modification recognition step. Yet, CAT-Seq uses <strong>Nanopore Sequencing</strong> for several reasons:</p> |
| − | <p class="listin">1. Nanopore sequencing allows to sequence<strong> long molecules</strong>, which eliminates the need of biomolecule fragmentation. This is an extremely important characteristic us | + | <p class="listin">1. Nanopore sequencing allows to sequence<strong> long molecules</strong>, which eliminates the need of biomolecule fragmentation. This is an extremely important characteristic to us because when working with large libraries of mutants in single droplets, fragmenting amplified DNA in those droplets means that all of those fragments in droplets would need to be barcoded. While in theory, this is a possible thing to do, it would require an extra merging and amplification step which would immensely decrease the accuracy of the activity determination. That is because in general, every step of the biotechnological method never is one hundred percent efficient. In turn, the lower amount of steps your system needs to take in order to produce results - the more accurate it will be. That is why reducing the number of steps in CAT-Seq by <strong>omitting fragmentation and barcoding</strong> was extremely important for us.</p> |
<p class="listin">2. Secondly, nanopore sequencing can detect and discriminate different modified nucleotides with ease, because nanopore can detect slight changes in nucleotide structure and assign a different value to that signal.</p> | <p class="listin">2. Secondly, nanopore sequencing can detect and discriminate different modified nucleotides with ease, because nanopore can detect slight changes in nucleotide structure and assign a different value to that signal.</p> | ||
| Line 430: | Line 457: | ||
| − | <img class="image_design" style="; display: block; margin-top: -2%; margin-bottom: 3%;" src=" | + | <img class="image_design" style="; display: block; margin-top: -2%; margin-bottom: 3%;" src="https://static.igem.org/mediawiki/2018/f/f9/T--Vilnius-Lithuania-OG--nanopore.jpeg"> |
<p style="margin-bottom: 2%">Using the same principle, billions of different catalytic biomolecule sequences and their corresponding activities can be recorded.</p> | <p style="margin-bottom: 2%">Using the same principle, billions of different catalytic biomolecule sequences and their corresponding activities can be recorded.</p> | ||
| − | <img class="image_design" style="; display: block; width: 70%; margin: auto; padding-bottom: 4%;" src=" | + | <img class="image_design" style="; display: block; width: 70%; margin: auto; padding-bottom: 4%;" src="https://static.igem.org/mediawiki/2018/c/c8/T--Vilnius-Lithuania-OG--activities_1.jpeg"> |
| Line 444: | Line 471: | ||
| − | <img class="image_design" style="; display: block; width: 80%; margin: auto; padding-bottom: 1%;" src=" | + | <img class="image_design" style="; display: block; width: 80%; margin: auto; padding-bottom: 1%;" src="https://static.igem.org/mediawiki/2018/9/93/T--Vilnius-Lithuania-OG--mutants.jpeg"> |
| Line 450: | Line 477: | ||
| − | <img class="image_design" style="; display: block; width: 80%; margin: auto; padding-bottom: 1%;" src=" | + | <img class="image_design" style="; display: block; width: 80%; margin: auto; padding-bottom: 1%;" src="https://static.igem.org/mediawiki/2018/7/73/T--Vilnius-Lithuania-OG--mut_act.jpeg"> |
| − | <p>To read about more about proof of concept results, please click here.</p> | + | <p>To read about more about proof of concept results, please <a href="https://2018.igem.org/Team:Vilnius-Lithuania-OG/Demonstrate">click here</a>.</p> |
</div> | </div> | ||
Latest revision as of 00:36, 18 October 2018