| (28 intermediate revisions by 5 users not shown) | |||
| Line 13: | Line 13: | ||
<link rel="stylesheet" type="text/css" | <link rel="stylesheet" type="text/css" | ||
href="https://2018.igem.org/wiki/index.php?title=Template:Hong_Kong_HKUST/main.css&action=raw&ctype=text/css"/> | href="https://2018.igem.org/wiki/index.php?title=Template:Hong_Kong_HKUST/main.css&action=raw&ctype=text/css"/> | ||
| + | <style> | ||
| + | .column { | ||
| + | float: left; | ||
| + | width: 33.33%; | ||
| + | padding: 5px; | ||
| + | } | ||
| + | /* Clear floats after image containers */ | ||
| + | .row::after { | ||
| + | content: ""; | ||
| + | clear: both; | ||
| + | display: table; | ||
| + | } | ||
| + | @media screen and (max-width: 500px) { | ||
| + | .column { | ||
| + | width: 100%; | ||
| + | } | ||
| + | } | ||
| + | </style> | ||
</head> | </head> | ||
<body style="background-color:black"> | <body style="background-color:black"> | ||
| Line 31: | Line 49: | ||
<header class="align-center"> | <header class="align-center"> | ||
| − | <center><img src="https://static.igem.org/mediawiki/2018/ | + | <center><img src="https://static.igem.org/mediawiki/2018/8/86/T--Hong_Kong_HKUST--MFCbanner.png" class="rounded mx-auto d-block" alt="..." width="100%" height="100%"></center> |
| − | < | + | < |
</header> | </header> | ||
</div> | </div> | ||
| Line 51: | Line 69: | ||
</header> | </header> | ||
<center> | <center> | ||
| − | <div style="text-align: center; width:100%; max-width:800px;" class="image fit" style="background-color: #fff; width:100%; max-width:600px; "><img src="https://static.igem.org/mediawiki/2018/a/ab/T--Hong_Kong_HKUST--MFCdesign.jpeg" class="img-fluid" alt="Responsive image"></div> | + | <div style="text-align:center; width:100%; max-width:800px;" class="image fit" style="background-color: #fff; width:100%; max-width:600px; "><img src="https://static.igem.org/mediawiki/2018/a/ab/T--Hong_Kong_HKUST--MFCdesign.jpeg" class="img-fluid" alt="Responsive image"></div> |
| − | </center> | + | </center><br> |
<p style="color:black;">Aside from plastic degradation and alkane metabolism, generation of electricity was another important focus of our iGEM project. For this module, we focused on generating a stable electrical current by utilizing <i>Shewanella oneidensis MR-1</i> strain’s inbuilt extracellular electron transport mechanism. In order to better harness its electrogenicity, we housed a culture of the bacterium within a microbial fuel cell of our design, aiming at maximizing electrical output for a given amount of substrate. | <p style="color:black;">Aside from plastic degradation and alkane metabolism, generation of electricity was another important focus of our iGEM project. For this module, we focused on generating a stable electrical current by utilizing <i>Shewanella oneidensis MR-1</i> strain’s inbuilt extracellular electron transport mechanism. In order to better harness its electrogenicity, we housed a culture of the bacterium within a microbial fuel cell of our design, aiming at maximizing electrical output for a given amount of substrate. | ||
</p> | </p> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | <p style="color:black;">While many other microorganisms are capable of electron transport by reduction of suitable substrate, we chose to work with <i>Shewanella oneidensis MR-1</i> strain as it is a relatively well-studied facultative anaerobe capable of generating current by reducing a broad range of substrates, utilizing them as metabolites. While the processes behind this electron transport mechanism are not yet fully understood, it is known that a few outer and inner-membrane Cytochromes are responsible for the shuttling of electrons from within the cell body to the extracellular substrate. The multiple heme-centres present within these Cytochromes allows for efficient transport of electrons to the extracellular space. The extracellular electron transport mechanism is described in figure 1. | |
| − | + | ||
| − | <p style="color:black;">While many other microorganisms are capable of electron transport by reduction of suitable substrate, we chose to work with <i>Shewanella oneidensis MR-1</i> strain as it is a relatively well-studied facultative anaerobe capable of generating current by reducing a broad range of substrates, utilizing them as metabolites. While the processes behind this electron transport mechanism are not yet fully understood, it is known that a few outer and inner-membrane Cytochromes are responsible for the shuttling of electrons from within the cell body to the extracellular substrate. The multiple heme-centres present within these Cytochromes allows for efficient transport of electrons to the extracellular space. | + | |
</p> | </p> | ||
<center> | <center> | ||
| − | <div style="text-align: center; width:100%; max-width: | + | <div style="text-align:center; width:100%; max-width:600px;" class="image fit" style="background-color: #fff; width:100%; max-width:600px; "><img src ="https://static.igem.org/mediawiki/2018/thumb/6/64/T--Hong_Kong_HKUST--EET.png/800px-T--Hong_Kong_HKUST--EET.png"> |
</div> | </div> | ||
| − | </center> | + | </center><br> |
| − | <p style="color:black;">According to literature, electrons are shuttled from the inner-membrane Cytochrome c molecule CymA to another Cytochrome c molecule, MtrA, present in the periplasm. They are then transported to the outer-membrane Cytochromes MtrC and OmcA. These outer-membrane cytochromes are in fact lipoproteins associated to the outer membrane and outer-membrane protein MtrB. By this arrangement, the outer-membrane Cytochromes are exposed to the extracellular space where they may reduce suitable substrates. This reduction may occur by direct contact between cell surface and substrate or may be mediated by Flavins to reduce substrate far from the cell surface. Riboflavins can mediate such indirect reduction of substrate because of their conjugated double bonded structures, allowing | + | <p style="color:black; text-align:center;"><b>Figure 1</b> Extracellular Electron Transport mechanism of <i>Shewanella oneidensis MR-1</i> |
| + | </p> | ||
| + | <p style="color:black;">According to literature<sup>[1]</sup>, electrons are shuttled from the inner-membrane Cytochrome c molecule CymA to another Cytochrome c molecule, MtrA, present in the periplasm. They are then transported to the outer-membrane Cytochromes MtrC and OmcA. These outer-membrane cytochromes are in fact lipoproteins associated to the outer membrane and outer-membrane protein MtrB. By this arrangement, the outer-membrane Cytochromes are exposed to the extracellular space where they may reduce suitable substrates. This reduction may occur by direct contact between cell surface and substrate or may be mediated by Flavins<sup>[2]</sup> to reduce substrate far from the cell surface. Riboflavins can mediate such indirect reduction of the substrate because of their conjugated double-bonded structures, allowing the small energy transitions carried by the electrons. Additionally, its large polar tail confers its significant solubility within the external medium to shuttle electrons to distant substrates. | ||
</p> | </p> | ||
| − | <p style="color:black;">Aside from its extensive electron transport pathways, Shewanella oneidensis MR-1 is particularly useful to our project from a microbial fuel cell perspective because of its ability to produce biofilms that facilitate close contact between the bacteria and the cell electrode. These biofilms are formed by an interconnected network of a type IV pili from the cell membrane that adheres to the solid substrate. The pili are conductive and function as nanowires that aid in the direct reduction of the substrate. It is however worth noting that more robust biofilm formation occurs in anaerobic and low-nutrient conditions. This was an important factor to consider while designing our microbial fuel cell as we had to decide the nutrient concentration of the culture medium for optimal cell growth and biofilm formation while maintaining an oxygen-free environment. | + | <p style="color:black;">Aside from its extensive electron transport pathways, <i>Shewanella oneidensis MR-1</i> is particularly useful to our project from a microbial fuel cell perspective because of its ability to produce biofilms that facilitate close contact between the bacteria and the cell electrode. These biofilms are formed by an interconnected network of a type IV pili from the cell membrane that adheres to the solid substrate. The pili are conductive and function as nanowires that aid in the direct reduction of the substrate<sup>[3]</sup>. It is, however, worth noting that more robust biofilm formation occurs in anaerobic and low-nutrient conditions. This was an important factor to consider while designing our microbial fuel cell as we had to decide the nutrient concentration of the culture medium for optimal cell growth and biofilm formation while maintaining an oxygen-free environment. |
</p> | </p> | ||
| − | <p style="color:black;">Having gone through many prototypes, we designed our own microbial fuel cell taking inspiration from those of past iGEM teams such as the 2013 Bielefeld team. For the body, we chose | + | |
| + | <center> | ||
| + | <div style="text-align: center; width:100%; max-width:600px;" class="image fit" style="background-color: #fff; width:100%; max-width:600px; "> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/5/5d/T--Hong_Kong_HKUST--MFC_V1.jpeg" class="img-fluid" alt="Responsive image"></div> | ||
| + | </center> | ||
| + | <br> | ||
| + | <p style="color:black; text-align:center;"><b>Figure 2</b> MFC design</p> | ||
| + | |||
| + | |||
| + | <p style="color:black;">Having gone through many prototypes, we designed our own microbial fuel cell taking inspiration from those of past iGEM teams such as the 2013 Bielefeld team<sup>[4]</sup>. We decided to employ 3D-printing as a fast and low-cost method to produce our own prototypes. For the body, we chose ABS (Acrylonitrile Butadiene Styrene) because it could not be degraded by <i>Shewanella oneidensis MR-1</i>, while is known to be non-toxic. In our experimental set-up, as shown in figure 3, the anode compartment housed the culture medium containing <i>Shewanella oneidensis MR-1</i> strain in anaerobic conditions. To achieve such an anaerobic environment, we initially considered flooding the chamber with inert nitrogen gas but later decided to use an anaerobic chamber due to limitations our lab faced with the usage of nitrogen gas. For the anode material, we decided to use carbon cloth as it has a high effective surface area and provides a suitable surface for the bacteria to tether onto and initiate biofilm formation. Meanwhile, the cathode compartment was aerobic in nature with Ferricyanide as the terminal electron acceptor and PBS buffer to maintain the pH constantly at 7 throughout the cell’s operation. We needed a proton-selective membrane to separate the anode and cathode compartments so that the circuit may be completed by the transfer of H+ ions from the anode to the cathode without allowing for the direct reduction of the terminal electron acceptor. For this, we chose to use a Nafion membrane due to its highly selective nature and efficiency in proton exchange. | ||
</p> | </p> | ||
| + | |||
| + | <center> | ||
| + | <div style="text-align:center; width:100%; max-width:600px;" class="image fit" style="background-color: #fff; width:100%; max-width:600px; "><img src ="https://static.igem.org/mediawiki/2018/thumb/7/7a/T--Hong_Kong_HKUST--MFC_expdesign.png/800px-T--Hong_Kong_HKUST--MFC_expdesign.png"> | ||
| + | </div> | ||
| + | </center><br> | ||
| + | <p style="color:black; text-align:center;"><b>Figure 3</b> Experimental MFC set-up</p> | ||
| + | |||
<p style="color:black;"> | <p style="color:black;"> | ||
| − | With the microbial fuel cell assembled and the bacteria introduced into the anode chamber, we | + | With the microbial fuel cell assembled and the bacteria introduced into the anode chamber, we began a series of tests to determine our output potential. The results of which are elaborated <a href="">here</a>. |
</p> | </p> | ||
| − | < | + | <section id="One" class="wrapper style3"> |
| + | <div class="inner"> | ||
| + | <header class="align-center"> | ||
| + | |||
| + | <h2>REFERENCES:</h2> | ||
| + | |||
| + | </header> | ||
| + | </div> | ||
| + | </section> | ||
| + | <p> <br/> | ||
[1]J. Myers and C. Myers, "Role for Outer Membrane Cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in Reduction of Manganese Dioxide", Applied and Environmental Microbiology, vol. 67, no. 1, pp. 260-269, 2001. <br/> | [1]J. Myers and C. Myers, "Role for Outer Membrane Cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in Reduction of Manganese Dioxide", Applied and Environmental Microbiology, vol. 67, no. 1, pp. 260-269, 2001. <br/> | ||
Latest revision as of 01:31, 18 October 2018


OVERVIEW

Aside from plastic degradation and alkane metabolism, generation of electricity was another important focus of our iGEM project. For this module, we focused on generating a stable electrical current by utilizing Shewanella oneidensis MR-1 strain’s inbuilt extracellular electron transport mechanism. In order to better harness its electrogenicity, we housed a culture of the bacterium within a microbial fuel cell of our design, aiming at maximizing electrical output for a given amount of substrate.
While many other microorganisms are capable of electron transport by reduction of suitable substrate, we chose to work with Shewanella oneidensis MR-1 strain as it is a relatively well-studied facultative anaerobe capable of generating current by reducing a broad range of substrates, utilizing them as metabolites. While the processes behind this electron transport mechanism are not yet fully understood, it is known that a few outer and inner-membrane Cytochromes are responsible for the shuttling of electrons from within the cell body to the extracellular substrate. The multiple heme-centres present within these Cytochromes allows for efficient transport of electrons to the extracellular space. The extracellular electron transport mechanism is described in figure 1.
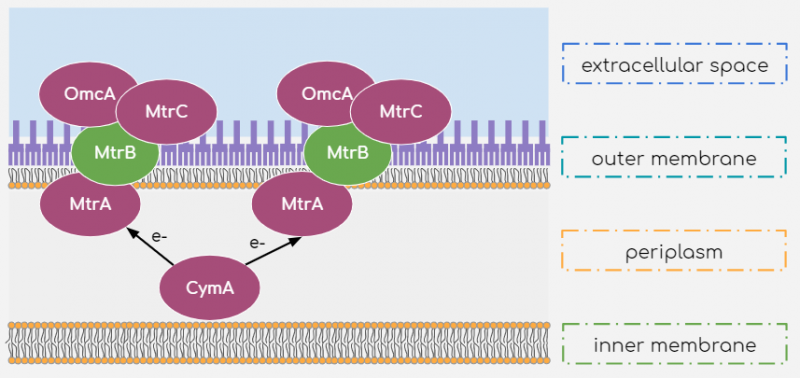
Figure 1 Extracellular Electron Transport mechanism of Shewanella oneidensis MR-1
According to literature[1], electrons are shuttled from the inner-membrane Cytochrome c molecule CymA to another Cytochrome c molecule, MtrA, present in the periplasm. They are then transported to the outer-membrane Cytochromes MtrC and OmcA. These outer-membrane cytochromes are in fact lipoproteins associated to the outer membrane and outer-membrane protein MtrB. By this arrangement, the outer-membrane Cytochromes are exposed to the extracellular space where they may reduce suitable substrates. This reduction may occur by direct contact between cell surface and substrate or may be mediated by Flavins[2] to reduce substrate far from the cell surface. Riboflavins can mediate such indirect reduction of the substrate because of their conjugated double-bonded structures, allowing the small energy transitions carried by the electrons. Additionally, its large polar tail confers its significant solubility within the external medium to shuttle electrons to distant substrates.
Aside from its extensive electron transport pathways, Shewanella oneidensis MR-1 is particularly useful to our project from a microbial fuel cell perspective because of its ability to produce biofilms that facilitate close contact between the bacteria and the cell electrode. These biofilms are formed by an interconnected network of a type IV pili from the cell membrane that adheres to the solid substrate. The pili are conductive and function as nanowires that aid in the direct reduction of the substrate[3]. It is, however, worth noting that more robust biofilm formation occurs in anaerobic and low-nutrient conditions. This was an important factor to consider while designing our microbial fuel cell as we had to decide the nutrient concentration of the culture medium for optimal cell growth and biofilm formation while maintaining an oxygen-free environment.

Figure 2 MFC design
Having gone through many prototypes, we designed our own microbial fuel cell taking inspiration from those of past iGEM teams such as the 2013 Bielefeld team[4]. We decided to employ 3D-printing as a fast and low-cost method to produce our own prototypes. For the body, we chose ABS (Acrylonitrile Butadiene Styrene) because it could not be degraded by Shewanella oneidensis MR-1, while is known to be non-toxic. In our experimental set-up, as shown in figure 3, the anode compartment housed the culture medium containing Shewanella oneidensis MR-1 strain in anaerobic conditions. To achieve such an anaerobic environment, we initially considered flooding the chamber with inert nitrogen gas but later decided to use an anaerobic chamber due to limitations our lab faced with the usage of nitrogen gas. For the anode material, we decided to use carbon cloth as it has a high effective surface area and provides a suitable surface for the bacteria to tether onto and initiate biofilm formation. Meanwhile, the cathode compartment was aerobic in nature with Ferricyanide as the terminal electron acceptor and PBS buffer to maintain the pH constantly at 7 throughout the cell’s operation. We needed a proton-selective membrane to separate the anode and cathode compartments so that the circuit may be completed by the transfer of H+ ions from the anode to the cathode without allowing for the direct reduction of the terminal electron acceptor. For this, we chose to use a Nafion membrane due to its highly selective nature and efficiency in proton exchange.

Figure 3 Experimental MFC set-up
With the microbial fuel cell assembled and the bacteria introduced into the anode chamber, we began a series of tests to determine our output potential. The results of which are elaborated here.
REFERENCES:
[1]J. Myers and C. Myers, "Role for Outer Membrane Cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in Reduction of Manganese Dioxide", Applied and Environmental Microbiology, vol. 67, no. 1, pp. 260-269, 2001.
[2]E. Marsili, D. Baron, I. Shikhare, D. Coursolle, J. Gralnick and D. Bond, "Shewanella secretes flavins that mediate extracellular electron transfer", Proceedings of the National Academy of Sciences, vol. 105, no. 10, pp. 3968-3973, 2008.
[3]M. El-Naggar, G. Wanger, K. Leung, T. Yuzvinsky, G. Southam, J. Yang, W. Lau, K. Nealson and Y. Gorby, "Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1", Proceedings of the National Academy of Sciences, vol. 107, no. 42, pp. 18127-18131, 2010.
[4]"Team:Bielefeld-Germany/Project/MFC - 2013.igem.org", 2013.igem.org, 2018. [Online]. Available: https://2013.igem.org/Team:Bielefeld-Germany/Project/MFC. [Accessed: 30- Aug- 2018].

