| Line 103: | Line 103: | ||
<div class="center"><img src="https://static.igem.org/mediawiki/2018/8/8d/T--Imperial_College--BiocontainmentXT.gif"></div> | <div class="center"><img src="https://static.igem.org/mediawiki/2018/8/8d/T--Imperial_College--BiocontainmentXT.gif"></div> | ||
</br> | </br> | ||
| − | <p1>A big socio-ethical issue with using genetically engineered organisms is the issue of biocontainment. We recognized this as an issue by talking to <a href="https://2018.igem.org/Team:Imperial_College/Public_Engagement#opinion"><b>members of the public</b></a> as well as from the <a href="https://2018.igem.org/Team:Imperial_College/Public_Engagement#discussion"><b>socio-ethics discussion</b></a>. These organisms should not be released where they could potentially cause ecological damage by outcompeting or harming native species. While some may debate the impact of this ecological damage, it would be easier to persuade governments and its people to use GMOs when proper biocontainment measures are in place. Public and governmental opposition to widespread implementation of synthetic biology products will greatly affect the downstream applications of our system. This problem rings especially true as (for now) EU laws and regulations require that any release of GMOs into the environment proper risk assessments and containment strategies must be in place | + | <p1>A big socio-ethical issue with using genetically engineered organisms is the issue of biocontainment. We recognized this as an issue by talking to <a href="https://2018.igem.org/Team:Imperial_College/Public_Engagement#opinion"><b>members of the public</b></a> as well as from the <a href="https://2018.igem.org/Team:Imperial_College/Public_Engagement#discussion"><b>socio-ethics discussion</b></a>. These organisms should not be released where they could potentially cause ecological damage by outcompeting or harming native species. While some may debate the impact of this ecological damage, it would be easier to persuade governments and its people to use GMOs when proper biocontainment measures are in place. Public and governmental opposition to widespread implementation of synthetic biology products will greatly affect the downstream applications of our system. This problem rings especially true as (for now) EU laws and regulations require that any release of GMOs into the environment proper risk assessments and containment strategies must be in place <a href="http://www.loc.gov/law/help/restrictions-on-gmos/eu.php" class="highlight" target="_blank">(LOC, 2015)</a>. By controlling transcription of growth retardants or toxins, like gp2 and MazF respectively, we can control where our bacteria will live and thus add a layer of biocontainment. |
</br></br> | </br></br> | ||
| Line 115: | Line 115: | ||
Placeholder for Fabric Bioprinter GIF | Placeholder for Fabric Bioprinter GIF | ||
</br> | </br> | ||
| − | <p1>In preparation for our <a href="https://2018.igem.org/Team:Imperial_College/Public_Engagement#art"><b>art exhibition</b></a>, we discussed the integration of science and art with a student, Alice, from the RCA. She mentioned that in fashion, chemical pollution as a result of the usage of dyes is prominent. Further reading made us aware that textile dyeing is the second largest polluter of clean water globally | + | <p1>In preparation for our <a href="https://2018.igem.org/Team:Imperial_College/Public_Engagement#art"><b>art exhibition</b></a>, we discussed the integration of science and art with a student, Alice, from the RCA. She mentioned that in fashion, chemical pollution as a result of the usage of dyes is prominent. Further reading made us aware that textile dyeing is the second largest polluter of clean water globally <a href="https://www.independent.co.uk/life-style/fashion/environment-costs-fast-fashion-pollution-waste-sustainability-a8139386.html">(Perry, 2018)</a>. We realized that using bacteria to synthesize dyes could provide for an ecologically friendly solution. Moreover, with the ability to pattern using our electrode array, we can design simple prints using MelA which is a step in the right direction for the fashion industry. We have also succeeded in cloning the MelA gene into our construct design. |
</br></br><div class="center"><img src="https://static.igem.org/mediawiki/2018/b/bf/T--Imperial_College--IHP3.png"></div> | </br></br><div class="center"><img src="https://static.igem.org/mediawiki/2018/b/bf/T--Imperial_College--IHP3.png"></div> | ||
<a class="btn btn-primary btn-lg" href="https://2018.igem.org/Team:Imperial_College/Demonstrate#expt9" role="button">Click here for experimental results</a></br> | <a class="btn btn-primary btn-lg" href="https://2018.igem.org/Team:Imperial_College/Demonstrate#expt9" role="button">Click here for experimental results</a></br> | ||
Revision as of 03:45, 17 October 2018
Integrated HP

Summary of Integrated Human Practices
Safety
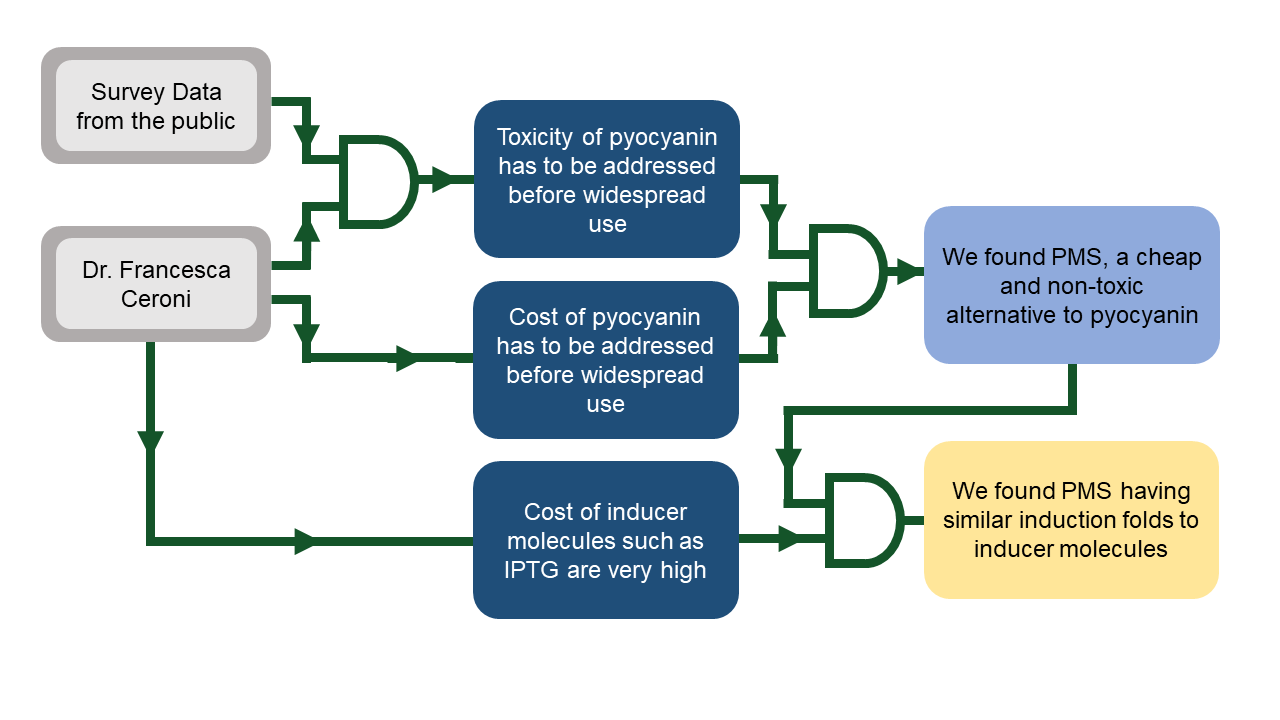
Toxicity comparison between Pyocyanin and PMS
The 2012 OSHA Hazard Communication Standard ranks hazard ratings with the use of categories, with Category 0 being the lowest risk and Category 4 being the highest. With regards to toxicity, pyocyanin is a Category 4 substance (Santa Cruz Biotechnology, 2010) and extreme care was taken during our wet lab to ensure our own safety and any contact with pyocyanin would warrant immediate medical attention, PMS on the other hand is a Category 0 substance (Santa Cruz Biotechnology, 2017) and thus is far easier and safer to handle.Cost comparison between PMS and common inducer molecules
A cursory look at the costs of PMS, pyocyanin and common inducer molecules (such as IPTG) already reveal stark differences in costs per gram. When accounting for working concentrations, this difference is further magnified, with PMS being 407 times cheaper than IPTG and 6600 times cheaper than pyocyanin. These costs are summarized in a table below, where costs per gram are obtained using the lowest price per gram on Sigma-Aldrich. However costs only matter if it can be shown that PMS can have a similar fold induction to common inducer molecules such as IPTG and experimental results for fold induction suggesting that is the case can be found below.| Inducer | Working Concentrations | Price per gram (£) | Mass per liter of media (mg) | Price per liter of media (pence) | CAS No. | Relative price to PMS (%) |
|---|---|---|---|---|---|---|
| PMS | 0.2 uM | 15.76 | 0.0613 | 0.0966 | 299-11-6 | n/a |
| Pyocyanin | 2.5 uM | 12,120 | 0.526 | 638 | 85-66-5 | 660,000 |
| IPTG | 40 uM (NEB, 2018) | 41.2 | 9.53 | 39.3 | 367-93-1 | 40,700 |
| L-Arabinose | 6.66 M (Spadiut et. al., 2010) | 0.785 | 1000 | 78.5 | 5328-37-0 | 81,300 |
| aTc | 0.214 uM (Nallamsetty and Waugh, 2007) | 1650 | 0.0991 | 16.4 | 13803-65-1 | 17,000 |

Environment
Biocontainment


Fabric Bioprinter
Placeholder for Fabric Bioprinter GIF
Wellbeing














