| Line 758: | Line 758: | ||
<p>Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.</p> | <p>Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.</p> | ||
</div> | </div> | ||
| − | + | <img src= "https://static.igem.org/mediawiki/2018/c/c1/T--Imperial_College--SBOL_table.png" alt="" width="28%";> | |
| + | |||
<div type="button" class="accordion">Relevance</div> | <div type="button" class="accordion">Relevance</div> | ||
<div class="panel expt6p"> | <div class="panel expt6p"> | ||
Revision as of 06:27, 17 October 2018
Results

Building and Testing Pixcell Constructs
Assembling the Pixcell Constructs
The PixCell Construct consists of a repurposed version of the soxRS regulon from E. coli, consisting of SoxR and GFP being expressed from either side of the pSoxR/pSoxS bidirectional promoter. pSoxR provides constitutive expression of SoxR. When oxidised, either directly by redox-cycling molecule or by oxidative stress, SoxR binds and activates transcription of GFP downstream of pSoxS.
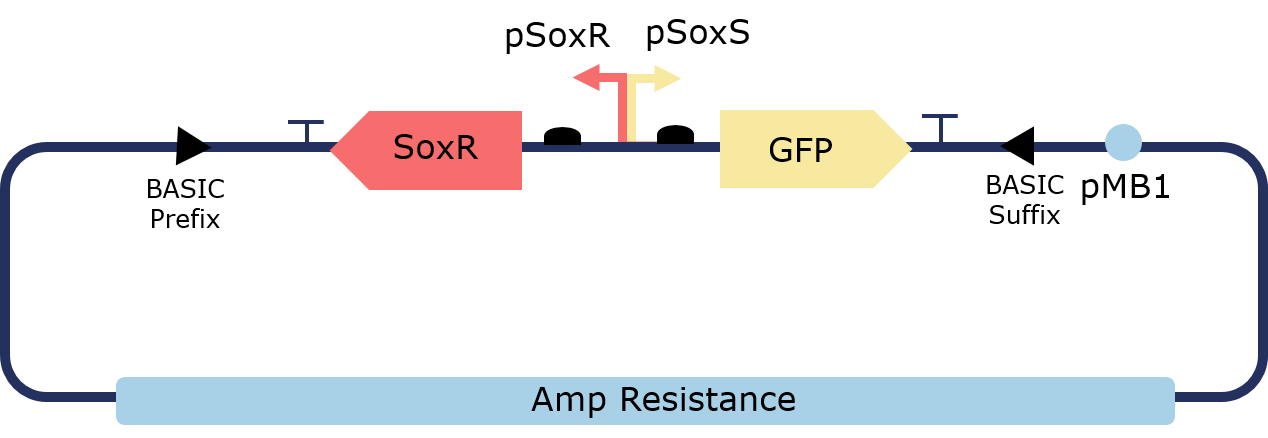
Figure 1. Illustration of the PixCell Construct
The Pixcell construct was designed to test whether we could control the expression of GFP by controlling the oxidation state of SoxR through our electrochemical set up.
Using the Golden Gate assembly method, with two inserts; the SOXRS regulon and GFP, which were originally amplified out from the E. coli genome and a storage vector respectively, the construct was assembled. Gel electrophoresis showed that a construct of approximately the right length was produced and the construct was then sequence verified.

Figure 1. PCR results from the amplification of the entire construct
In order to lower the basal level of GFP expression a degradation tag was added. Therefore, any increase in GFP expression induced by the oxidation of SoxR would be more predominant.

Figure 1. Illustration of the PixCell Construct DegTag
The Pixcell construct enables us to test whether we can control gene expression by controlling the oxidation state of soxR. The Pixcell construct DegTag enables the system to be more dynamic in that seeing the system switch from on to off is quicker.
Characterizing Pixcell Constructs
We characterised the PixCell circuits in a series of steps in order to make them respond to an electronic input. In order to demonstrate if the PixCell construct is responsive to oxidation signals, we started by chemically inducing the circuit with oxidised pyocyanin in solution, as at the time we did not have an electrode setup that would work aerobically in solid cultures. Characterization experiments were performed by measuring cell growth and GFP expression at a range of increasing pyocyanin, ferricyanide and ferrocyanide concentrations, as fully described in the “Methods” section.
These results demonstrate that the assembled PixCell circuit can be switched on by oxidative signals and switched off by reductive signals. These experimental data were also handed over the dry lab, where information about fold induction and degradation rates supported and further implemented the theoretical models.
As a first step, we investigated the effect of oxidised pyocyanin on cell health. We observed that pyocyanin concentrations higher than 10 uM significantly impacted cell health (figure 1). We then measured GFP expression at a range of different concentrations (figure 2). We found an optimal trade-off between cell health and GFP expression at a concentration of 2.5 uM, which is half of that used by (Tschirhart et al., 2017) in their anaerobic system (figure 3). We hypothesised that a reason for this lower concentration might be because in our aerobic system less oxidising input is required. Once identified our working pyocyanin concentration, that is the cellular redox-odulator, we varied the concentration of ferro/ferricyanide, the redox-modulators at the electrode interface, to find an optimal redox balance between pyocyanin and the reduced and oxidised forms of FCN (figure 5, 6, 7, 8) . As above, we investigated the effect of the redox molecules on cell health and identified the a FCN concentration (10 mM), which provided optimal balance between GFP expression and cell health.
Figure 1.Pyocyanin affects cell viability




Concentrations of pyocyanin higher than 10 uM significantly affects cell viability. E. coli DJ901 (soxR mutant) growth is rescued by PicXell Circuits 1 and 2, which encode the redox-sensitive SoxR protein. Positive and negative control (no soxR) are more susceptible to oxidative stress
Figure 2.Pyocyanin induces GFP expression




As expected, oxidated pyocyanin induces GFP expression in E. coli DJ901 transformed with PixCell Circuit 1 and 2. Describe here region at 10 uM and why optimal is 2.5 uM
Figure 3.Pyocyanin dose response

GFP expression in E. coli DJ901 with PixCell Circuits 1 and 2 is higher than the positive control. As expected, the PixCell Circuit 2 (with GFP-degradation tag) has lower GFP/OD600 values in the steady state regions.




Compared to pyocyanin, the addition of the reducing agent sodium sulfite, seems to protect the cells from oxidative stress due to the addition of oxidised ferricyanide in solution
Figure 5.Ferricyanide induces GFP expression in the presence of sodium sulfite in solution




The concentration of sodium sulfite is constant, whereas ferricyanide is increased. The PixCell circuits are turned on only after a concentration of sulphite higher than x...
Figure 6.Ferrocyanide does not affect cell growth in the presence of sodium sulfite in solution




Addition of reduced ferrocyanide and sodium sulphite maintains the system in a reduced state. This rescues cell viability compared to addition of oxidised ferricyanide, probably due to a decrease in oxidative stress in solution.
Figure 7.Ferrocyanide does not induce GFP expression in solution




System is in reduced state so circuit is off
Figure 8.Ferro/ferricyanide dose responses


As expected, addition of oxidised ferricyanide in the presence of constant concentration of sulfites maintain the system off. Oxidised ferricyanide on the other side induces GFP expression in a sigmoidal fashion. This result proves that by being able to interconvert between the two species, we are able to to selectively turn on and off the system.
Figure 3.Pyocyanin dose response

GFP expression in E. coli DJ901 with PixCell Circuits 1 and 2 is higher than the positive control. As expected, the PixCell Circuit 2 (with GFP-degradation tag) has lower GFP/OD600 values in the steady state regions.
These experiments allowed us to demonstrate that our constructs are responsive to oxidising input and enabled us to identify the concentrations of redox-molecule. These informations enabled us to formulate the chemical compositions for the next experiments in aerobic solid cultures.
Electrochemistry of Redox Modulators
To demonstrate the ability of our electrode set-up to selectively oxidise and reduce our redox modulators in solution, we investigated the redox status of the our redox modulators with cyclic voltammetry (CV) and by measuring the absorbance at 390 nm with a plate reader. CV data showed that a voltage of +0.5 at the working electrode enables bulk oxidation, whereas a voltage of -0.4 allows bulk reduction. However, the presence of oxygen in aerobic environments interferes with the oxidation signal carried by the electrodes. Measuing optical absorbance at 390 nm with a plate reader, we then investigated the redox kinetics of pyocyanin in the presence of the reducing agent sodium sulfite.
These results show that our set-up is able to selectively and reversibly oxidise and reduce our redox modulators. Furthermore , by performing these electrochemistry experiments, we proved that addition of the reducing agent sodium sulfite enables complete chemical reduction of the system, even in aerobic environment.

Figure 1. Square-wave voltammetry setup
The above setup was used to perform Square-wave voltammetry to investigate bulk oxidation and reduction of our system. The setup was controlled using a multichannel potentiostat connected to a computer.

Figure 2. Square-wave voltammetry of redox modulator
The graphs above summarise the results showing that our system was consistently bulk reduced at -0.3V and bulk oxidised at +0.5V. The addition of Sulfite and cell containing the pixCell construct did not significantly alter the reduction and oxidation profile. These results led us to chose +0.5V as our oxidising potential in the following experiments testing the spatial electronic control of gene induction.

Figure 3. Amperometry of the final plate conditions
Amperometry was performed on plates containing 2.5uM Pyocyanin, 10mM FCN(R), Ampicillin, Kanamycin, 0.03% sulfite and E. coli containing pixCell construct Deg Tag. The results show that the current decays exponentially until a steady state is reached indicating bulk oxidation of redox modulators.

Figure 4. Pyocyanin reduction by the reducing agent Sodium Sulfite
In order to chemically reduce our system, we added sodium sulfite as a reducing agent. We measured the concentration of oxidised pyocyanin in solutions with increasing sulfite concentration. As expected, the more sulphite added, the less absorption at 390 nm occurs (peak for oxidised pyocyanin). However this linear relationhiop resulted to be true only up to ca. 5% of sulphite. After that point it seems that an oxidised species is being produced.

Figure 5.Production of Pyocyanin Sulfonate at 2.5% Sulfite
To dig deeper into the reasons of the unexpected non-linear relationship described above, we played with lower sulfite concentration. After searching in the literature (Glasser, 2017), we hypothesised that sulfite could react with pyocyanin, producing pyocyanin sultanate. because pyocyanin sulfonate has an absorption peak at 400 nm, we performed the same experiment as above but this time measuring the OD at both 390 and 400 nm. We found that at a concentration of 2.5 % of sulfite, there is appear of absorption at 400 nm, suggesting that pyocyanin sultanate is being produced. Because pyocyanin sulfonate is toxic to cells, , we carried the next experiments with lower sulfite concentration.
These results describe the first ever aerobic electrogenetic control system, that works both in liquid and solid E. coli cultures.
Spatial Electronic Control of Gene Induction
Here we demonstrated the first spatial activation of gene expression using electronic control.
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.
An electrode rig was set up to apply these potentials to cells grown on an agar plate containing the final reaction condition of 2.5μM pyocyanin, 0.02% sodium sulfite and 10mM ferrocyanide. Redox reactions only occur at the electrode surface during an electrochemistry experiment, meaning that oxidised pyocyanin and ferrocyanide were only produced in close proximity to the working electrode upon the application of a +0.5V pulse. Fluorescence images of agar plates clearly show localised expression of GFP around the electrode. This not only shows that the device allows for electronic control of gene expression, but also demonstrates high spatial control meaning it can be used for programmable spatial patterning of cell populations.

Figure 1. Setup of the electrode rig for the electronic control of spatial gene induction

Figure 2. Image of the electrode rig placed in the incubator.
Figures 1 and Image 2 illustrate the setup of the electrode rig. The working, counter and reference electrodes are penetrating the plate through holes. Cells were plated onto an agar plate containing the final working concentrations: 10mM FCN(R), 2.5 uM pyocyanin, 0.03% sulfite, ampicillin and kanamycin. Agar is fully covering the electrodes which are controlled by a potentiostat. An oxidising potential of +0.5% was applied.

Figure 3. Expected outcome for the electronic control of gene expression on an agar plate
Figure 3 illustrates the expected increased fluorescence around the working electrode. The oxidising potential results in bulk oxidation and activation of our system.
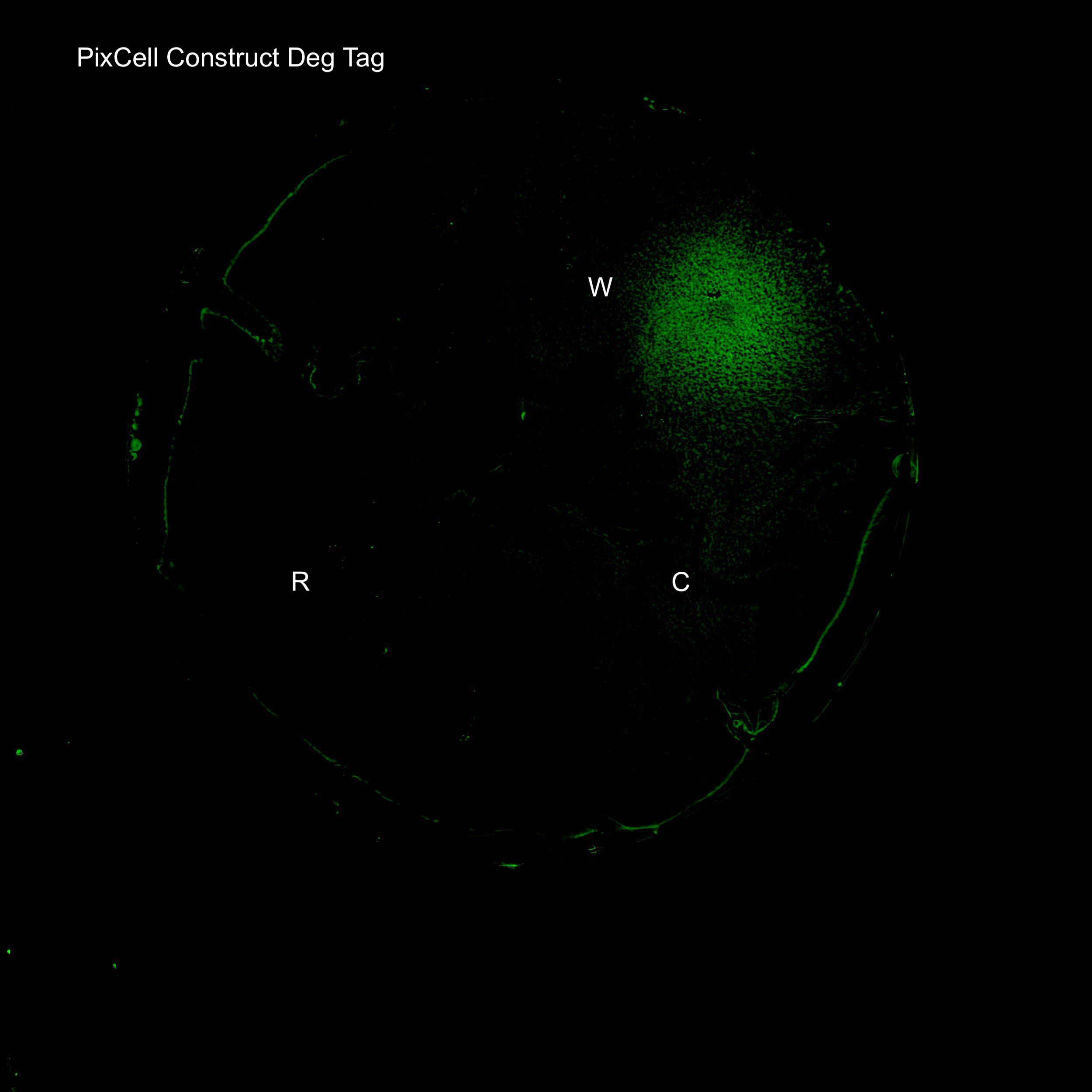
Figure 4. GFP expression after electric stimulation
We demonstrated elevated GFP expression around the working electrode in figure 4.

Figure 5. Comparison of PixCell Construct, PixCell Construct Deg Tag, positive control and negative control after application of an oxidising potential
We next investigated GFP expression of PixCell Construct and PixCell Construct Deg Tag relative to the negative control (DJ901) and the positive control which constitutively expresses GFP. Fluorescence image analysis shows increased fluorescence around the working electrodes demonstrating electronic control of spatial gene expression. The addition of a degradation tag to GFP substantially reduces background fluorescence.
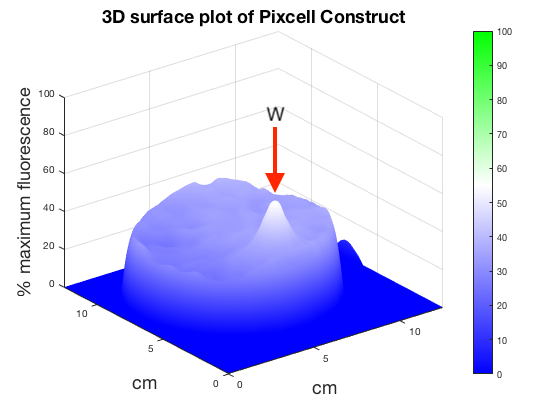



Figure 6. 3D surface plot showing relative fluorescence of PixCell Construct, PixCell Construct Deg Tag, positive control and negative control after electric stimulation
To better visualise the results 3D surface plots were made showing GFP expression in the z axis. GFP expression level from the positive and negative control was used to set a relative scale of GFP expression. Peaks around the working electrodes clear indicate increased GFP expression. PixCell construct Deg Tag showed lower background fluorescence compared to PixCell construct.

Figure 7. Comparison of PixCell Construct, PixCell Construct Deg Tag, positive control and negative control after application of a reducing potential
Next the 4 cell types were exposed to a reducing potential of -0.3V. Working electrodes do not show increased fluorescence. Higher background fluorescence in the plate with cells containing PixCell Construct compared to the the previous fluorescence images exposed to an oxidising potential is due to variation in spreading.

Figure 8. GFP expression on a plate with final conditions in the absence of pyocyanin
After successfully inducing spatially controlled activation of gene expression the system was tested in the absence of the redox modulator pyocyanin. The plate contains 10mM FCN(R), 0.03% sulfite, Ampicillin, Kanamycin. Results show that increased expression levels of GFP can be achieved around the working electrode in the absence of pyocyanin. Further fine tuning of the system will be required to achieve the level of precision obtained on a plate containing our final working conditions.
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.
Building and Characterizing the Pixcell Library
Creating the Sox Library
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat. Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.

Figure 1. PCR results of genomic extraction of SoxR from E. coli (including bi-directional promoter and SoxR protein gene)
Lorem ipsum dolor sit, amet consectetur adipisicing elit. Excepturi, nisi illum. Consequuntur cum, sequi quo, esse facere corrupti voluptatum ipsum vel consequatur error quasi tenetur, repellat voluptatibus aspernatur optio mollitia! Lorem ipsum dolor sit, amet consectetur adipisicing elit. Excepturi, nisi illum. Consequuntur cum, sequi quo, esse facere corrupti voluptatum ipsum vel consequatur error quasi tenetur, repellat voluptatibus aspernatur optio mollitia!
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.
Characterising the Sox Library
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.

Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat. Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.

Figure 1. PCR results of genomic extraction of SoxR from E. coli (including bi-directional promoter and SoxR protein gene)
Lorem ipsum dolor sit, amet consectetur adipisicing elit. Excepturi, nisi illum. Consequuntur cum, sequi quo, esse facere corrupti voluptatum ipsum vel consequatur error quasi tenetur, repellat voluptatibus aspernatur optio mollitia! Lorem ipsum dolor sit, amet consectetur adipisicing elit. Excepturi, nisi illum. Consequuntur cum, sequi quo, esse facere corrupti voluptatum ipsum vel consequatur error quasi tenetur, repellat voluptatibus aspernatur optio mollitia!
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.
Testing Alternative Non-toxic Redox Modulators
Testing Phenazine Methosulfate
Our electrogenetic control system relies on the electron carrying action of the redox-cycling drug pyocyanin, which is toxic metabolite from synthesised by Pseudomonas aeruginosa. This poses limitations to our system, with regards to safety and possible applications. Therefore, we searched in the literature for alternative SoxR inducers and and tested potential non-toxic alternatives. Shown below are the characterization data for the best candidate alternative: phenazine, methosulfate.
These results demonstrate the first ever use of PMS as an electrogenetic inducer.
The above setup was used to perform Sqaure-wave voltammetry confirming that ferrocyanide was bulk reduced at -0.3V and bulk oxidised at +0.5V.


Figure 1. Phenazine as as Cheaper, Non-toxic Redox Modulator
This working concentration provided an X-fold increase in GFP expression. Previous research suggests this response could be improved ~10-fold further by supplementing the growth media with branched-chain amino acids, the synthesis of which is inhibited by PMS leading to cell death.
These results demonstrates the SoxR/pSoxS system that we devised, can be used a non-toxic and effective tool for chemical, as well as electronic, gene induction. PMS is also incredibly cheap, being 40,000x cheaper than pyocyanin, ~190x cheaper than arabinose and ~29x cheaper than aTc per reaction.
Applications
Biocontainment- Gp2 Growth Inhibition
We used the Gp2 toxin to create an electrogenetic device for biocontainment applications. Electronic activation of the SoxR/pSoxS transcription factor - promoter couple can inhibit cell growth by inducing expression of Gp2, a T7 phage-derived RNA polymerase inhibitor. The construct was assembled into a plasmid with a low-copy number pSC101 origin of replication to reduce the effect of promoter leakiness impacting on cell growth.
These results serve as proof of concepts for the biocontainment application, as described in the “Applications” sections
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat. Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.
Figure 2.Negative controls
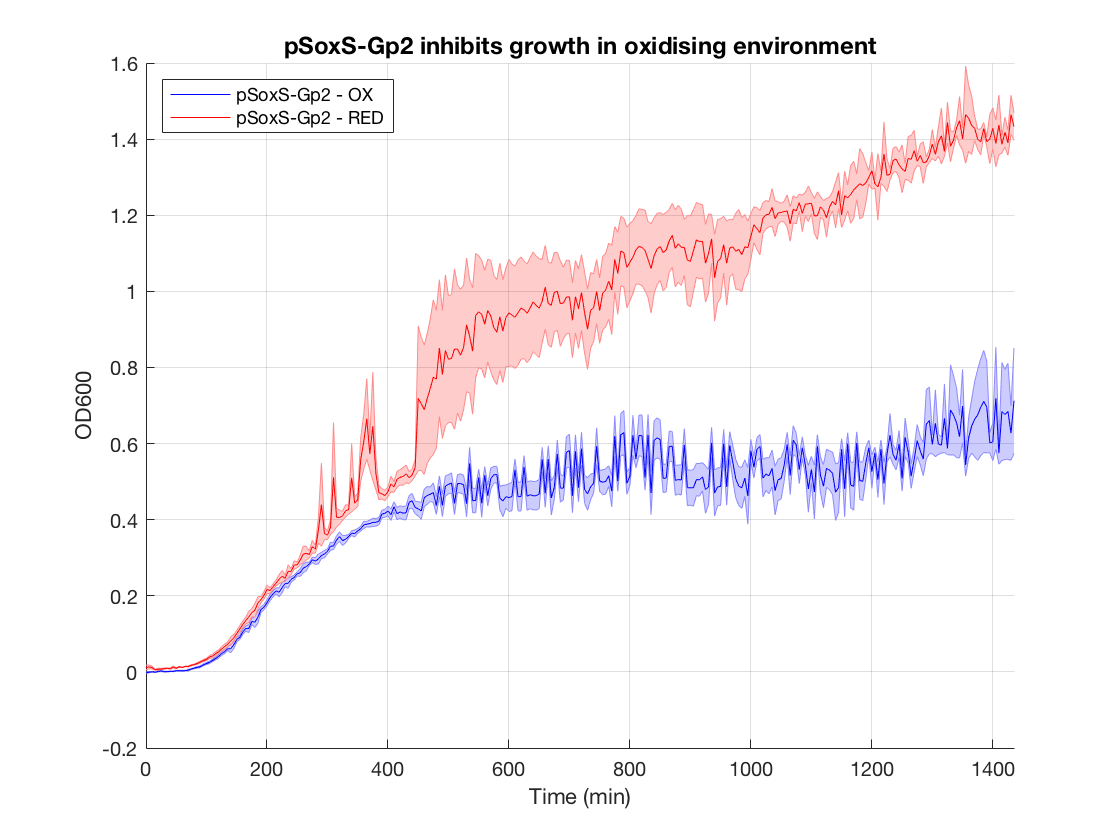
Figure 2.Negative controls

Figure 2.Negative controls

Explain results here.
With some improvement, and when used in combination with the PixCell electrode array, this biocontainment device could be used for biocontainment of GMOs in contained-use devices. This is analogous to how electric fences can be used to restrain livestock.
Fabric Bioprinting - Melanin
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat. Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.

Figure 1. PCR results of genomic extraction of SoxR from E. coli (including bi-directional promoter and SoxR protein gene)
Lorem ipsum dolor sit, amet consectetur adipisicing elit. Excepturi, nisi illum. Consequuntur cum, sequi quo, esse facere corrupti voluptatum ipsum vel consequatur error quasi tenetur, repellat voluptatibus aspernatur optio mollitia! Lorem ipsum dolor sit, amet consectetur adipisicing elit. Excepturi, nisi illum. Consequuntur cum, sequi quo, esse facere corrupti voluptatum ipsum vel consequatur error quasi tenetur, repellat voluptatibus aspernatur optio mollitia!
Lorem ipsum dolor, sit amet consectetur adipisicing elit. Recusandae dolore, tenetur expedita in architecto, repellat rerum minima cumque exercitationem debitis laborum fugit aliquam quo molestiae. Alias eius nostrum aspernatur quaerat.













