| Line 189: | Line 189: | ||
14.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, et al. (May 2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. The New England Journal of Medicine 358 (21): 2240–8. <br></p> | 14.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, et al. (May 2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. The New England Journal of Medicine 358 (21): 2240–8. <br></p> | ||
</div> | </div> | ||
| − | <div class="next_div"> <a href="https://2018.igem.org/Team:NJU-China/Results"><img class=" | + | <div class="next_div"> <a href="https://2018.igem.org/Team:NJU-China/Results"><img class="next_p" src="https://static.igem.org/mediawiki/2018/6/64/T--NJU-China--2018--Next.png" /></a></div> |
<div class="More" align="center" style=""> | <div class="More" align="center" style=""> | ||
<img src="https://static.igem.org/mediawiki/2018/a/a6/T--NJU-China--footer-img2.png" style="height: 150px;margin:auto" /> | <img src="https://static.igem.org/mediawiki/2018/a/a6/T--NJU-China--footer-img2.png" style="height: 150px;margin:auto" /> | ||
Revision as of 17:01, 17 October 2018




MOTIVATION
Neuron has different body parts, the soma, the dendrite, and the axon. Each part of the cell varies in the kinds and amounts of certain proteins and thus has different functions. Diseases might appear if, for some reasons, there is a lack of certain proteins in dendrites or axons. The goal of our work is to reverse the symptoms of those diseases in a safer, easier, more efficient and durable way to help people in need. The idea coming up is all about targeting, both inside and outside of the cell. First, we picked up a cis-acting RNA element, which can be transported to the neurites with the help of the RNA transporting system existing inside the cell. And if the element is connected with sequence of deficient proteins, it could be able to increase the deficient ones in neurites since proteins can be translated there directly. Secondly, we need a delivery system through which our elements are eventually meeting neurons and being transported into them after injection. Exosomes and AAV, able to carry our elements and target the neurons, are going to be tested for their efficiency.
Guidance Inside the Cell: UTRs
Why UTRs?
The neuronal granule system is a pathway neurons use to transport certain proteins to different cell parts. In this system, neuronal granules with RBPs binding to specified mRNA could be transported to neurites along microtubes in a kinesin-dependent manner. And translation begins in neurites after the granule finishes its job. We can see that this system offers us a method of RNA targeting. The 5’-UTR or 3’UTR of mRNA, which is recognized by neuronal granules, caught our attention. We believe it is an easier way to make use of the system by adding certain UTRs as RNA guide into our element.
Which UTRs?
As we know, generally it is the 3’-UTR of the mRNA recognized by neuronal granules that guides the mRNA to its destination. Due to the limited capacity of the two packaging systems, exosome, and AAV, and to expand application, we need the UTR sequence to be as short as possible so that longer protein sequences could be linked with our element. This is the reason we choose 3’-UTR of β-actin which only contains about 600 bases as one of our subjects.
Fortunately, according to recent studies, 5’-UTR of TBEV genomic RNA is able to hijack the neuronal granule system, which attracts our interest because it is only constituted by 131 bases. Obviously, it’s better to be part of the element. So it also becomes our experimental subject.
Does this 5’-UTR work?
We transferred three plasmids containing mCherry with or without UTRs into the neuron. First of all, we checked the cell viability when the RNA element was fused to plasmids.

And then, we compared the intensity of red fluorescence of mCherry in the neurites to solve the problem whether UTRs help to increase the amount of deficient proteins in neurites. Furthermore, to prove it is the RNA being transported, in situ hybridization was conducted with mCherry’s mRNA.
In detail, we constructed three plasmids in total. The first one, acting as the blank control, is pmR-mCherry vector. The second one, pmR-TBEV 5’-UTR -mCherry and the third one, pmR-mCherry-β-actin 3’-UTR, are constructed to verify whether the 3’-UTR and 5’-UTR could work and compare the efficiency of them. If in the second and third groups, the fluorescence of mCherry and in situ hybridization are of higher intensity in neurites than the control, it proves the UTRs do help to increase proteins in the neurites through the process of mRNA transporting.



Packages Targeting Neuron: Exosome and AAV
UTRs only function inside the cell, which means we need our elements to be delivered into neurons. Thus, packages able to cover our elements and transport them across neuron membrane are required.
Considering our team has been focused on exosome targeting for several years. Also, due to some of its advantages, such as brain accessibility, low immunogenicity, and specificity at recognizing certain cells, we believe it could be used as an element package. We collected exosomes secreted from 293T cells transfected with our plasmids and tested the quantity and size of the exosomes with NTA and the concentration of the mRNA with qPCR to determine the efficiency of the exosome package respectively. We found that elements with TBEV 5’-UTR decreased the mRNA level in exosomes significantly, this result suggests that exosome is not a suitable carrier for TBEV 5’-UTR RNAs.
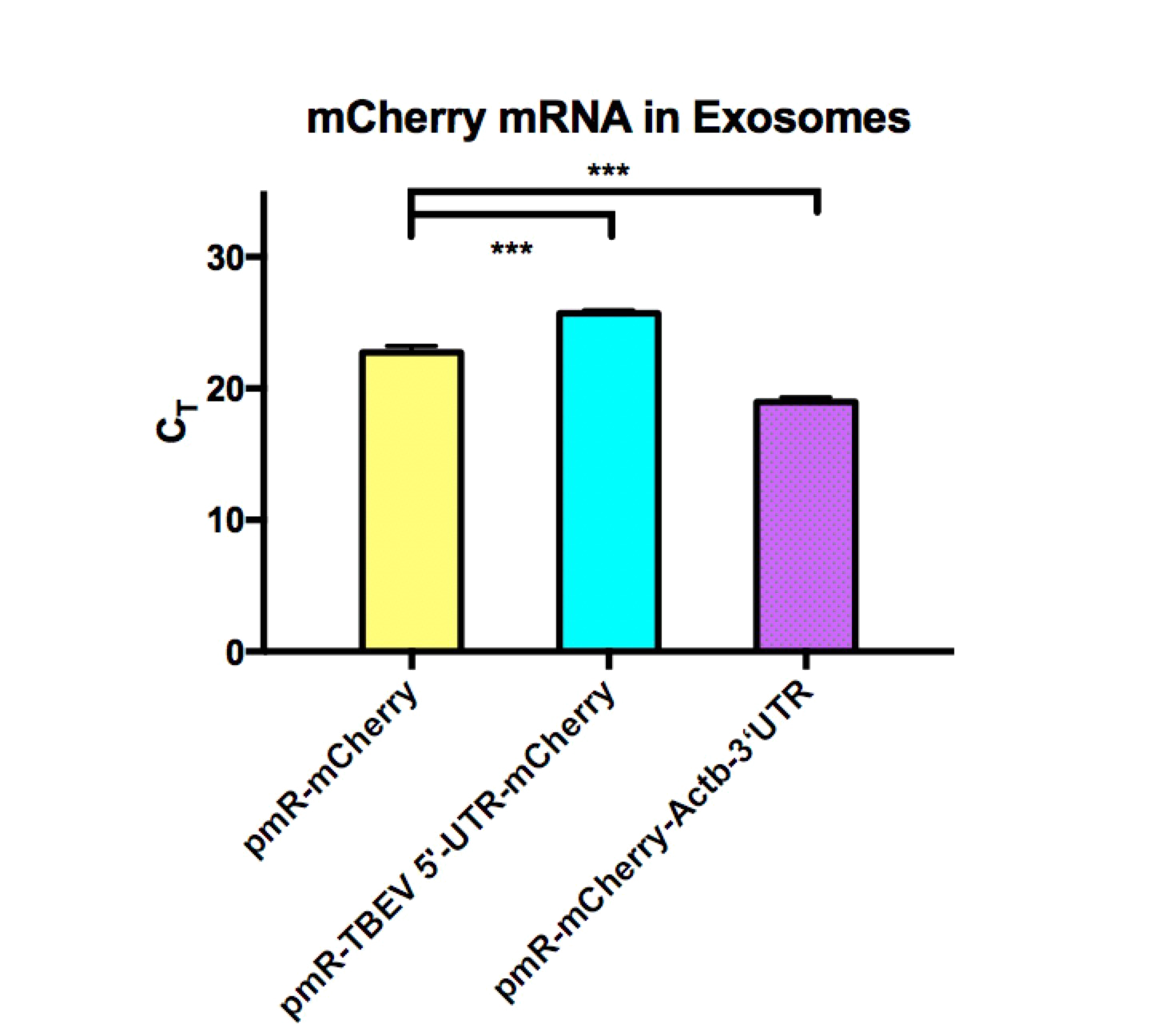
Nevertheless, we know that adeno-associated Virus (AAV) has been widely used by gene therapy researchers for not only the advantages of exosomes mentioned before but the high specificity of covering the element we want and the long period of gene expression. More importantly, it is not currently known to cause diseases. Based on the above, AAV seems to be an ideal package with vast potential for clinical use. We injected AAV covering mCherry with or without TBEV 5’-UTR into mice brain and detected the package preliminarily in 3 weeks.

References:
1.Wang F, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012, 14:22–29.
2.Bassell G. J., Zhang H., Byrd A. L., Femino A. M., Singer R. H., Taneja K. L., et al. . (1998). Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J. Neurosci. 18, 251–265.
3.Glock C., Heumüller M., Schuman E. M. (2017). mRNA transport and local translation in neurons. Curr. Opin. Neurobiol. 45, 169–177.
4.Hirano M., Muto M., Sakai M., Kondo H., Kobayashi S., Kariwa H., Yoshii K. Dendritic transport of tick-borne flavivirus RNA by neuronal granules affects development of neurological disease. Proc. Natl. Acad. Sci. USA. 2017;114:9960–9965
5.Giovanni Neri and Pietro Chiurani, X-linked Mental Retardation, 1999, Advances in Genetics, VoI. 41
6.Samulski RJ, Muzyczka N. 2014. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol 1:427–451.
7.Zhou Y, Zhou G, Tian C, Jiang W, Jin L, Zhang C, et al. Exosome-mediated small RNA delivery for gene therapy. Wiley Interdiscip Rev RNA (2016) 7(6):758–71.10.1002/wrna.1363
8.Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543
9.An JJ, et al. Distinct Role of Long 3′ UTR BDNF mRNA in Spine Morphology and Synaptic Plasticity in Hippocampal Neurons. Cell. 2008;134:175–187
10.Minato Hirano, Kentaro Yoshiia, et al. (2017) Dendritic transport of tick-borne flavivirus RNA by neuronal granules affects development of neurological disease. PNAS 114 (37) :9960-9965.
11.Salinas S, Schiavo G, Kremer EJ (2010) A hitchhiker’s guide to the nervous system: The complex journey of viruses and toxins. Nat Rev Microbiol 8:645–655.
12.Gould EA, Solomon T (2008) Pathogenic flaviviruses. Lancet 371:500–509
13.Deyle DR, Russell DW (August 2009) Adeno-associated virus vector integration. Current Opinion in Molecular Therapeutics, 11 (4): 442–7.
14.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, et al. (May 2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. The New England Journal of Medicine 358 (21): 2240–8.


