heading
Our Interlab
July 11th
Calibration 1:
We strictly followed the instructions and got our correction factor:
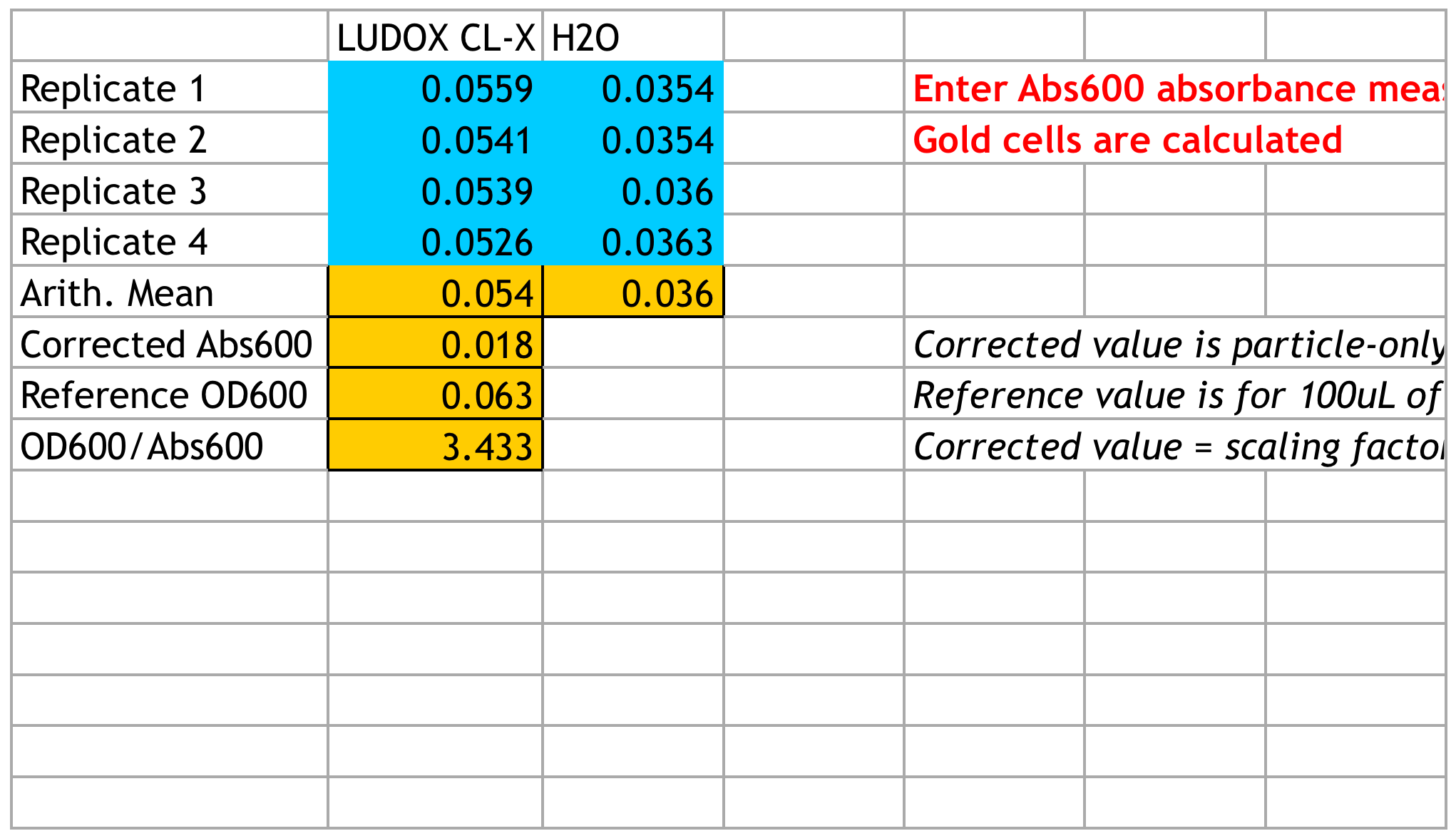

Calibration 2:
We prepared the microsphere stock solution and performed the serial dilution. Here is our result after measuring the Abs600:
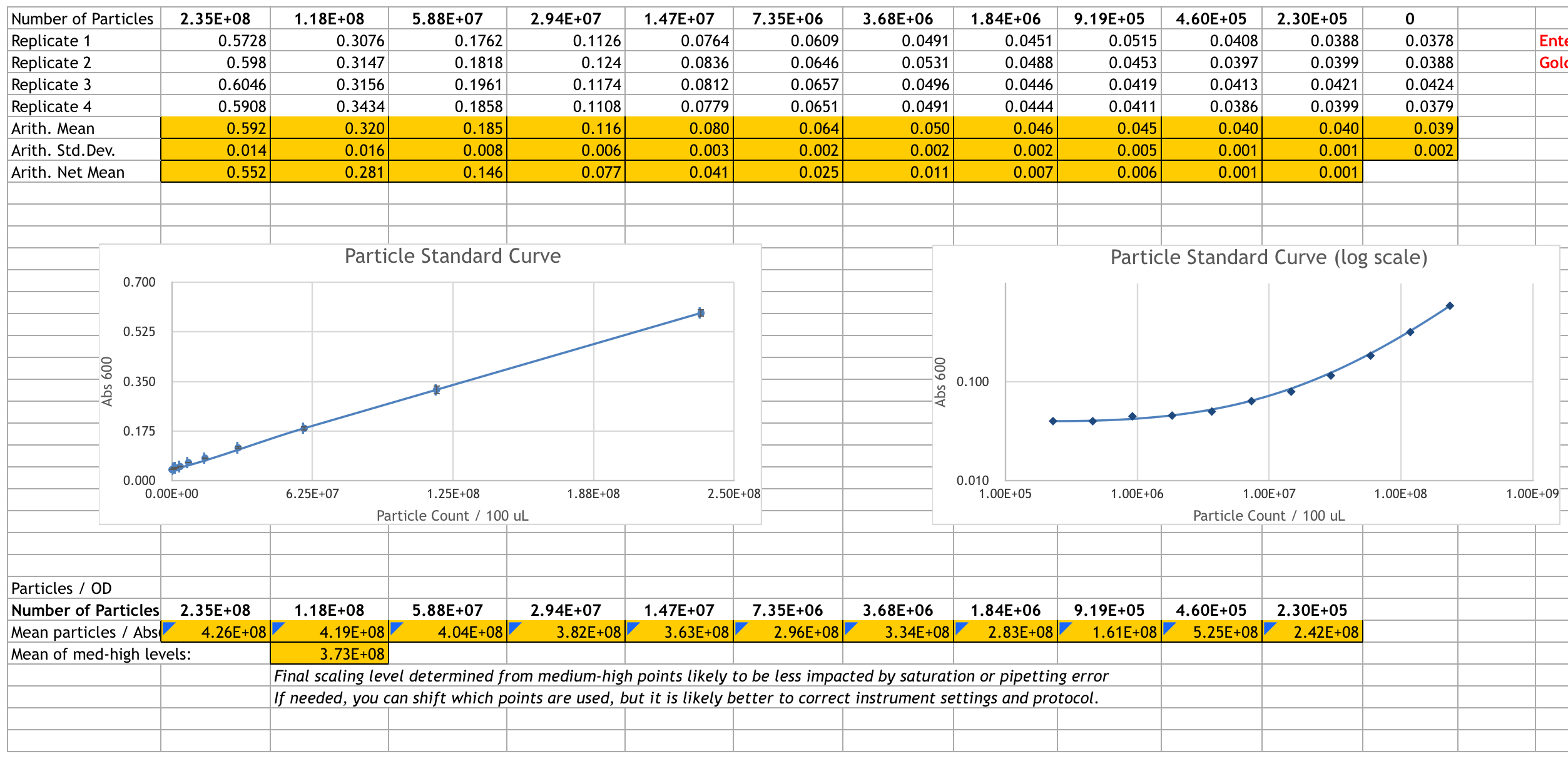
Calibration 3:
We prepared the fluorescein stock solution and performed the serial dilution. Here is our result after measuring the fluorescence:
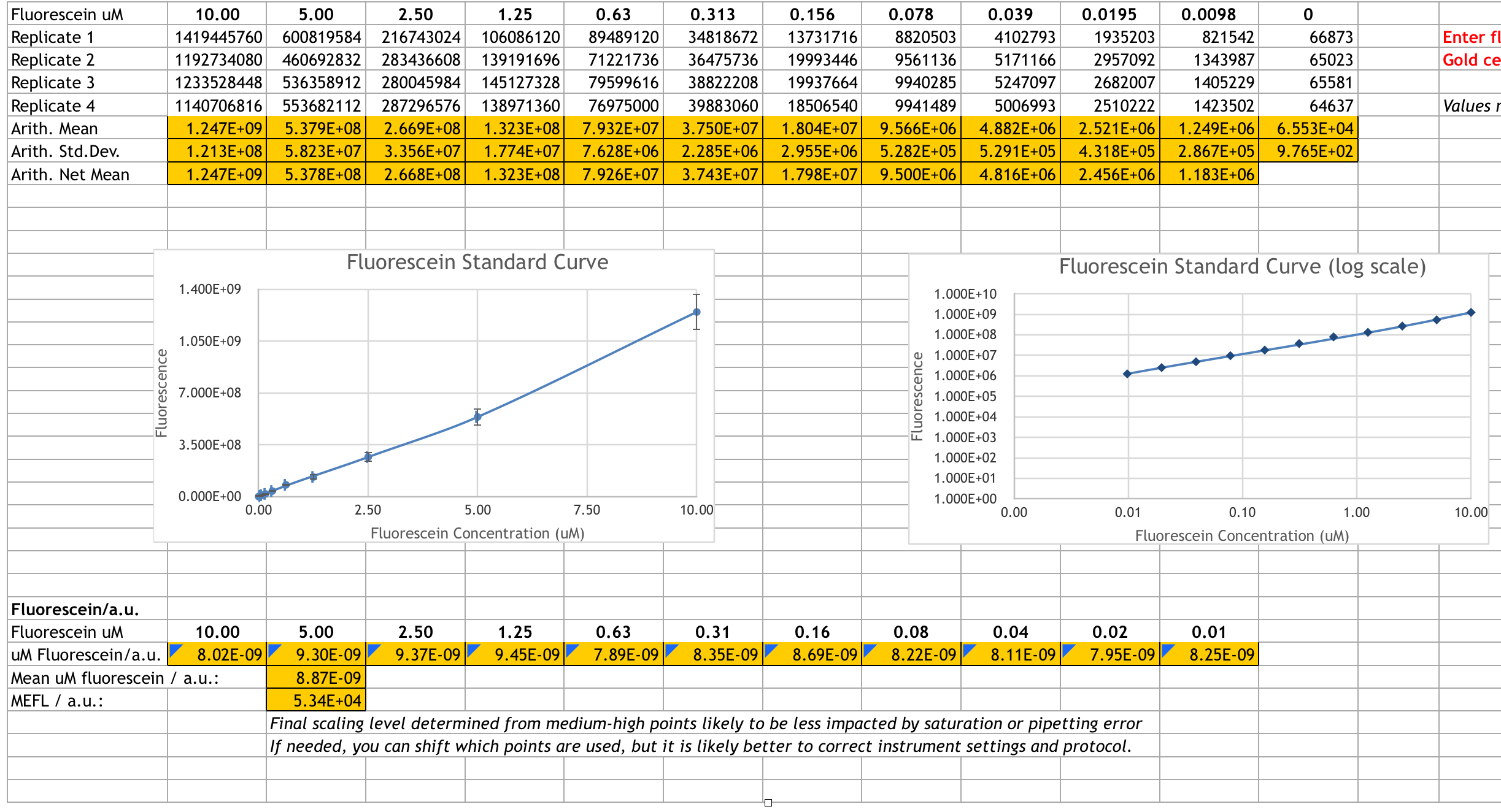
We performed the cell measurement and CFU from July 11th to July 15th
Cell Measurement Protocol:
We transformed Escherichia coli DH5α with the required plasmids on the first day and incubated them overnight. The second day we picked two colonies from each plate (16 total), inoculated them with LB+Cam, and grew them overnight at 37°C and 200 rpm. On the third day, we diluted our overnight culture to Abs600 0.2 in LB + Cam mixing a total of 12 mL solution. We then measured the absorbance and fluorescence values using SpectraMax i3 at 0 hour and at 6 hours following inoculation at 37°C and 200 rpm. Here is our results:
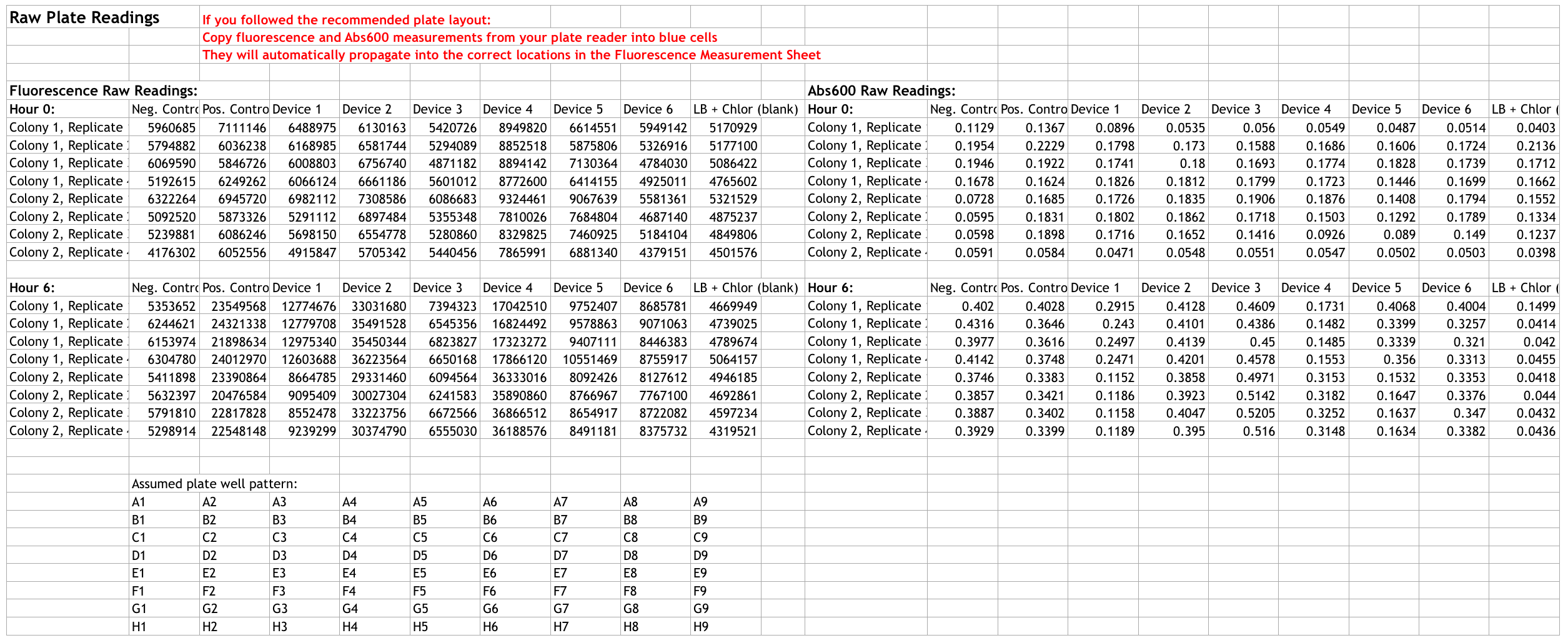
CFU:
After growing our cultures from positive and negative control overnight, we 8-fold diluted our culture and further diluted them to have 0.1 OD600. We then diluted the solutions more following the protocols using 2.0 mL and 1.5 mL tubes.

(Team member Yi kuang working at a clean bench)
Following up, we carefully spead the dilutions on previously prepared plates, marked them accordingly, and incubated them at 37°C overnight. However, when we were trying to count the colonies the day after, we were surprised by the amount of colonies grown.

(Plates from our first try)
Subsequently, we figured out that plates with 300 or more colonies are expected but we decided to perform this experiment again anyway to make sure our procedures were all as precise as possible. We then spent July 14th and July 15th to redo the CFU. Within our expectations, the overall data came out much better than the first time and we felt satisfied with the extra time put in.

