Kangyuan yu (Talk | contribs) |
|||
| (48 intermediate revisions by 13 users not shown) | |||
| Line 168: | Line 168: | ||
<ul class="dropdown-menu"> | <ul class="dropdown-menu"> | ||
<li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Modeling overview">Modeling overview</a></li> | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Modeling overview">Modeling overview</a></li> | ||
| − | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/ | + | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/model_of_systems">Model of systems</a></li> |
<li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Sort of three genes">Sort of three genes</a></li> | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Sort of three genes">Sort of three genes</a></li> | ||
| − | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Software"> | + | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Software">Software</a></li> |
</ul> | </ul> | ||
</li> | </li> | ||
| Line 186: | Line 186: | ||
<a href="#" data-toggle="dropdown" class="dropdown-toggle waves-effect waves-dark">HP<b class="caret"></b></a> | <a href="#" data-toggle="dropdown" class="dropdown-toggle waves-effect waves-dark">HP<b class="caret"></b></a> | ||
<ul class="dropdown-menu"> | <ul class="dropdown-menu"> | ||
| − | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/ | + | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Human Practices">Human Practices</a></li> |
| − | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/ | + | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Education_Engagement">Education&Engagement</a></li> |
</ul> | </ul> | ||
</li> | </li> | ||
| Line 193: | Line 193: | ||
<a href="#" data-toggle="dropdown" class="dropdown-toggle waves-effect waves-dark">TEAM<b class="caret"></b></a> | <a href="#" data-toggle="dropdown" class="dropdown-toggle waves-effect waves-dark">TEAM<b class="caret"></b></a> | ||
<ul class="dropdown-menu"> | <ul class="dropdown-menu"> | ||
| − | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Team | + | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Team">Team Members</a></li> |
<li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Collaborations">Collaborations</a></li> | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Collaborations">Collaborations</a></li> | ||
<li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Attributions">Attributions</a></li> | <li><a class="waves-effect waves-dark" href="https://2018.igem.org/Team:HUST-China/Attributions">Attributions</a></li> | ||
| Line 219: | Line 219: | ||
</div> | </div> | ||
</section> | </section> | ||
| + | <section style="margin-top: 10px;"> | ||
| + | <div class="container"> | ||
| + | <video id="my-video" class="video-js" controls preload="auto" width="100%" height="auto" | ||
| + | poster="https://static.igem.org/mediawiki/2018/8/83/T--HUST-China--2018-menu-vedio-poster01.png" data-setup="{}"> | ||
| + | <source src="https://static.igem.org/mediawiki/2018/7/7a/T--HUST-China--2018-menu-vedio04.webm" type="video/webm"> | ||
| + | <source src="https://static.igem.org/mediawiki/2018/4/4e/T--HUST-China--2018-menu-vedio03.mp4" type="video/mp4"> | ||
| + | <p class="vjs-no-js"></p> | ||
| + | <p class="vjs-no-js"> | ||
| + | To view this video please enable JavaScript, and consider upgrading to a web browser that | ||
| + | <a href="https://videojs.com/html5-video-support/" target="_blank">supports HTML5 video</a> | ||
| + | </p> | ||
| + | </video> | ||
| + | </div> | ||
| + | </section> | ||
<section class="content"> | <section class="content"> | ||
| Line 225: | Line 239: | ||
<div class="col-md-12 content-text"> | <div class="col-md-12 content-text"> | ||
<div class="about-logo"> | <div class="about-logo"> | ||
| − | <p>To convert optical energy into electric energy in a clean and sustainable way, Optopia is designed as a photovoltaic system consisting of photosynthetic microorganism (Rhodopseudomonas palustris) and electrogenic microorganism (Shewanella oneidensis). Synthetic biology strategies are applied to the system to trigger production and export of lactate in | + | <p> |
| − | + | To convert optical energy into electric energy in a clean and sustainable way, Optopia is designed as a photovoltaic system consisting of two subsystems: photosynthetic microorganism system (Synechocystis sp. or Rhodopseudomonas palustris) and electrogenic microorganism system (Shewanella oneidensis). Synthetic biology strategies are applied to the system to trigger production and export of lactate in photosynthetic microorganisms, as well as to improve efficiency of lactate utilization and extracellular electron generation in electrogenic microorganism system. </p> | |
| − | + | <br/> | |
| − | + | <p> | |
| − | + | Synechocystis, one kind of cyanobacteria, is more mature than Rhodopseudomonas in lactate production, but generating lots of oxygen during photosynthesis. Given the fact that Shewanella favors anaerobic environment for electricity production, Rhodopseudomonas may serve as a better carbon resource provider for Shewanella, not only because of its anaerobic photosynthesis maintaining an anaerobic environment required for extracellular electron generation in Shewanella, but also due to its capacity of reusing the waste from Shewanella. Hence, we construct a Synechocystis- Shewanella MFC and a Rhodopseudomonas - Shewanella MFC to find an optimized version of Optopia, maximzing the conversion of optical energy to electric energy. | |
| − | + | ||
| + | </p> | ||
| + | <div class="row"> | ||
| + | </div> | ||
| + | |||
| + | |||
<h3><strong>Part1: <span class="red-content">Photosynthetic microorganism system</span></strong></h3> | <h3><strong>Part1: <span class="red-content">Photosynthetic microorganism system</span></strong></h3> | ||
| − | + | </div> | |
| + | </div> | ||
</div> | </div> | ||
<div class="row"> | <div class="row"> | ||
<div class="col-md-12 info-blocks"> | <div class="col-md-12 info-blocks"> | ||
| − | <i class="icon-info-blocks material-icons | + | <i class="icon-info-blocks material-icons"><img class="img-responsive" src="https://static.igem.org/mediawiki/2018/8/8d/T--HUST-China--2018-lanzao01.png"></i> |
<div class="info-blocks-in" style="background-color: #ffffff; border: 1px solid #eeeeee;border-radius:5px;"> | <div class="info-blocks-in" style="background-color: #ffffff; border: 1px solid #eeeeee;border-radius:5px;"> | ||
| − | <h3>1. | + | <h3><b>1.1 Synechocystis sp.</b></h3> |
| − | <p>Synechocystis | + | <p>Synechocystis sp. PCC 6803 is often chosen as engineering microorganism to convert optical energy into chemical energy. In our project, we chose Synechocystis PCC6803 for optical energy conversion. Synechocystis sp. PCC 6803 produces lactate for the electricity production of Shewanella. However, Synechocystis sp. PCC 6803 itself lacks a pathway for producing lactate, and as a photoautotrophic microorganism, Synechocystis sp. PCC 6803 lacks a lactate transporter to transport lactate out of the cell[1]. Therefore, we have designed two strategies to modify Synechocystis sp. PCC 6803.</p> |
<div class="row"> | <div class="row"> | ||
</div> | </div> | ||
| − | + | <div class="col-md-6 col-md-offset-2" style="text-align: center;"> | |
| + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/6/64/T--HUST-China--2018-syndes2-picture.png"> | ||
| + | </div> | ||
<div class="col-md-12"> | <div class="col-md-12"> | ||
<p>Strategy 1:</p> | <p>Strategy 1:</p> | ||
| − | <p>The lactate dehydrogenase gene and the lactate transporter gene are combined in one circuit to achieve lactate production and transportation. For lactate dehydrogenase gene, we chose ldhD, | + | <p>The lactate dehydrogenase gene and the lactate transporter gene are combined in one circuit to achieve lactate production and transportation. For lactate dehydrogenase gene, we chose ldhD, while ldhDc is a codon-optimized version of ldhD, ldhDnARSdR is ldhD with D176A/I177R/F178S/N180R, and ldhDARSdR is the codon-optimized version of ldhDnARSdR. These codon optimizations are aimed at increasing the production of lactate. The lldP protein gene is used to transport the lactate out of the cell. The lldP protein has 12 transmembrane alpha-helical segments and generally lacks cleaved signal sequences. Besides, it cotransports lactate with a proton[1]. In order to detect the expression of these two genes, we add the Flag and 6 × His sequences respectively after the two.</p> |
| + | </div> | ||
| + | <div class="col-md-6 col-md-offset-3" style="text-align: center;"> | ||
| + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/b/be/T--HUST-China--2018-experimental_1-ldhD-lldP.png"> | ||
| + | <p>Figure 1. circuit of ldhD-lldP.</p> | ||
| + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/c/ce/T--HUST-China--2018-experimental_2-ldhDC-lldP.png"> | ||
| + | <p>Figure 2. circuit of ldhDC-lldP.</p> | ||
| + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/a/a9/T--HUST-China--2018-experimental_3-ldhDnARSdR-lldP.png"> | ||
| + | <p>Figure 3. circuit of ldhDnARSdR-lldP.</p> | ||
| + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/b/ba/T--HUST-China--2018-experimental_4-ldhDARSdR-lldP.png"> | ||
| + | <p>Figure 4. circuit of ldhDARSdR-lldP.</p> | ||
| + | |||
</div> | </div> | ||
<div class="col-md-12"> | <div class="col-md-12"> | ||
<p>Strategy 2:</p> | <p>Strategy 2:</p> | ||
| − | <p>In response to the preference for NADPH in the metabolism of Synechocystis, transhydrogenase gene is used to achieve the goal of producing more NADPH, and the glycerol dehydrogenase gene is used to increase the lactate production. For transhydrogenase gene, we use TH | + | <p>In response to the preference for NADPH in the metabolism of Synechocystis sp. PCC 6803, transhydrogenase gene is used to achieve the goal of producing more NADPH, and the glycerol dehydrogenase gene is used to increase the lactate production. For transhydrogenase gene, we use TH and gldA for glycerol dehydrogenase. The gene lldP is also used to transport the lactate out of the cell. And the same as strategy 1, the Flag and 6 × His sequences are used to detect the expression of the two genes.</p> |
</div> | </div> | ||
| − | + | <div class="col-md-6 col-md-offset-3" style="text-align: center;"> | |
| − | + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/c/c4/T--HUST-China--2018-experimental_5-TH-gldA-lldP.png"> | |
| − | + | <p>Figure 5. circuit of TH-gldA-lldP.</p></div> | |
| − | + | ||
<div class="col-md-12"> | <div class="col-md-12"> | ||
| − | <p>After successfully constructing these gene circuits, the shuttle plasmid | + | <p>After successfully constructing these gene circuits, the shuttle plasmid pCK306 is used to transform the genes into Synechocystis sp. PCC 6803, and the yellow fluorescent protein gene on the pCK306 plasmid is used to detect the transformation.</p> |
</div> | </div> | ||
| − | |||
</div> | </div> | ||
| + | </div> | ||
| + | </div> | ||
| − | + | ||
| − | + | <div class="row"> | |
| − | <i class="icon-info-blocks material-icons | + | <div class="col-md-12 info-blocks"> |
| + | <i class="icon-info-blocks material-icons"><img class="img-responsive" src="https://static.igem.org/mediawiki/2018/6/63/T--HUST-China--2018-zhaozehong01.png"></i> | ||
<div class="info-blocks-in" style="background-color: #ffffff; border: 1px solid #eeeeee;border-radius:5px;"> | <div class="info-blocks-in" style="background-color: #ffffff; border: 1px solid #eeeeee;border-radius:5px;"> | ||
| − | <h3> | + | |
| − | <p>Rhodopseudomonas palustris is a kind of Purple Non-sulfur Bacteria. Under anaerobic conditions, Rhodopseudomonas palustris can utilize hydrogen, sodium thiosulfate, hydrogen sulfide as electron donors for photoautotrophic growth. Furthermore, it can also have heterotrophic growth under microaerobic to aerobic conditions [2]. In addition, Rhodopseudomonas palustris is widely used in sewage treatment | + | |
| − | + | <div class="col-md-12"> | |
| − | <div class="col-md-12"> | + | <h3><b>1.2 Rhodopseudomonas palustris<b/></h3> |
| − | <p>To enhance the production of lactate in Rhodopseudomonas palustris, we plan to promote the conversion efficiency of pyruvate to D-lactate and malate to L-lactate. Therefore, we decide to transfer these two genes, mleS [5] and ldhA, [6] into Rhodopseudomonas palustris to generate more lactate intracelluarly. Considering the necessity of transporting lactate out of the cells, we also apply the gene lldP [7] encoding lactate permease.Since the order of these three genes in a polycistronic lead to different expression levels [8], we build a model to optimize the gene order to maximize the gene expression. Also, we use codon optimized tool Jcat [9] to optimize the codons of these genes.</p> | + | <p>Rhodopseudomonas palustris is a kind of Purple Non-sulfur Bacteria. Under anaerobic conditions, Rhodopseudomonas palustris can utilize hydrogen, sodium thiosulfate, hydrogen sulfide as electron donors for photoautotrophic growth. Furthermore, it can also have heterotrophic growth under microaerobic to aerobic conditions [2]. In addition, Rhodopseudomonas palustris is widely used in sewage treatment because it is environmental friendly and accessible [3]. Since it is capable of maintaining an anaerobic environment and reusing the acetate generated by Shewanella, Rhodopseudomonas palustris may be a better carbon source provider for Shewanella compared with Synechocystis sp. PCC 6803. |
| − | + | ||
| − | + | Therefore, we decided to modify Rhodopseudomonas palustris so that it could produce lactate under the anaerobic condition and export it out of the cell. According to KEGG database [4], the metabolic pathways related to lactate metabolism in Rhodopseudomonas palustris are as follows: .</p></div> | |
| − | <div class="col-md-12"> | + | <div class="col-md-7 col-md-offset-2" > <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/d/da/T--HUST-China--2018-rhododes-picture.png"></div> |
| − | <p>mleS:malate dehydrogenase, the conversion of malic acid to L-lactate.</p> | + | |
| − | + | ||
| − | + | ||
| − | + | <div class="col-md-12"> | |
| − | + | ||
| − | + | <p>To enhance the production of lactate in Rhodopseudomonas palustris, we plan to promote the conversion efficiency of pyruvate to D-lactate and malate to L-lactate. Therefore, we decide to transfer these two genes, mleS [5] and ldhA, [6] into Rhodopseudomonas palustris to generate more lactate intracelluarly. Considering the necessity of transporting lactate out of the cells, we also apply the gene lldP [7] encoding lactate permease. | |
| − | + | ||
| − | + | Since the order of these three genes in a polycistronic lead to different expression levels [8], we build a model to optimize the gene order to maximize the gene expression. Also, we use codon optimized tool Jcat [9] to optimize the codons of these genes.</p> | |
| − | + | </div> | |
| + | <div class="col-md-7 col-md-offset-2" > <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/0/00/T--HUST-China--2018-description-picture3.png.png"></div> | ||
| + | |||
| + | <div class="col-md-12"> | ||
| + | |||
| + | <p>mleS:malate dehydrogenase, the conversion of malic acid to L-lactate. | ||
| + | </p> | ||
| + | <p>ldhA:fermentative D-lactate dehydrogenase, NAD-dependent, convert pyruvate to D-lactate.</p> | ||
| + | <p>lldP:L-lactate permease, the lactate is transported out of the cell.</p> | ||
| + | <p>To validate our modeling results, we continue to build three different gene circuits which can determine the highest lactate production efficiency of our total circuit:</p> | ||
| + | </div> | ||
| + | <div class="col-md-7 col-md-offset-2" > <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/0/0d/T--HUST-China--2018-description-picture4.png.png"></div> | ||
| + | |||
| + | <div class="col-md-7 col-md-offset-2" > <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/6/63/T--HUST-China--2018-description-picture5.png.png"></div> | ||
| + | <div class="col-md-7 col-md-offset-2" > <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/8/88/T--HUST-China--2018-description-picture6.png.png"></div> | ||
</div> | </div> | ||
</div> | </div> | ||
| − | </div> | + | </div> |
| − | + | <div class="row"> | |
| − | + | <div class="col-md-12 content-text"> | |
| − | + | <div class="about-logo"> | |
| + | |||
| + | |||
| + | |||
<h3><strong>Part2: <span class="red-content">Electrogenic microorganism system</span></strong></h3> | <h3><strong>Part2: <span class="red-content">Electrogenic microorganism system</span></strong></h3> | ||
</div> | </div> | ||
| − | + | </div> | |
</div> | </div> | ||
<div class="row"> | <div class="row"> | ||
<div class="col-md-12 info-blocks"> | <div class="col-md-12 info-blocks"> | ||
| − | <i class="icon-info-blocks material-icons | + | <i class="icon-info-blocks material-icons"><img class="img-responsive" src="https://static.igem.org/mediawiki/2018/0/0c/T--HUST-China--2018-xiwa01.png"></i> |
<div class="info-blocks-in" style="background-color: #ffffff; border: 1px solid #eeeeee;border-radius:5px;"> | <div class="info-blocks-in" style="background-color: #ffffff; border: 1px solid #eeeeee;border-radius:5px;"> | ||
| − | <h3> | + | <h3><b>Shewanella oneidensis<b/></h3> |
<p>Shewanella oneidensis is becoming more and more popular these years as it could transfer electron to the electrode and generate electricity. This fascinating power mainly attributes to a number of conductive c-type cytochromes (c-Cyts) including OmcA-MtrCAB and CymA. CymA could conduct electrons to the MtrCAB complex. Then, the bacteria would use its extension or vesicles of the outer membrane and periplasm to transfer outer membrane c-type cytochromes.</p> | <p>Shewanella oneidensis is becoming more and more popular these years as it could transfer electron to the electrode and generate electricity. This fascinating power mainly attributes to a number of conductive c-type cytochromes (c-Cyts) including OmcA-MtrCAB and CymA. CymA could conduct electrons to the MtrCAB complex. Then, the bacteria would use its extension or vesicles of the outer membrane and periplasm to transfer outer membrane c-type cytochromes.</p> | ||
<div class="col-md-6 col-md-offset-2" style="text-align: center;"> | <div class="col-md-6 col-md-offset-2" style="text-align: center;"> | ||
| Line 321: | Line 373: | ||
</div> | </div> | ||
<div class="col-md-6 col-md-offset-2" style="text-align: center;"> | <div class="col-md-6 col-md-offset-2" style="text-align: center;"> | ||
| − | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/ | + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/e/e4/T--HUST-China--2018-shewades2-picture.png"> |
</div> | </div> | ||
<div class="col-md-12"> | <div class="col-md-12"> | ||
| Line 352: | Line 404: | ||
</div> | </div> | ||
</div> | </div> | ||
| − | + | ||
<div class="row"> | <div class="row"> | ||
| − | + | <div class="col-md-12 content-text"> | |
| − | + | <div class="about-logo"> | |
| − | + | ||
| − | + | <h3><strong>Part3: <span class="red-content">Whole design</span></strong></h3> | |
| − | + | </div> | |
| − | + | </div> | |
| + | </div> | ||
<div class="row"> | <div class="row"> | ||
<div class="col-md-12 info-blocks"> | <div class="col-md-12 info-blocks"> | ||
| − | <i class="icon-info-blocks material-icons | + | <i class="icon-info-blocks material-icons"><img class="img-responsive" src="https://static.igem.org/mediawiki/2018/9/98/T--HUST-China--2018-coin17.png"></i> |
<div class="info-blocks-in" style="background-color: #ffffff; border: 1px solid #eeeeee;border-radius:5px;"> | <div class="info-blocks-in" style="background-color: #ffffff; border: 1px solid #eeeeee;border-radius:5px;"> | ||
| − | <h3>Design of | + | <h3><b>Design of MFC(Microbial Fuel Cell)<b/></h3> |
| − | <p>We have designed a bipolar chamber MFC this year. Proton exchange membrane divided it into anode chamber and cathode chamber. Anode chamber containing S.oneidensis, nutrient substance(LB、lactate ) or other electrical producing microbes were sealed to prevent the entry of external oxygen. Considering safety and oxidation-reduction potential, we put ferric chloride solution in cathode chamber so that S.oneidensis can transfer electrons outside of their membranes by electron transport chain. Then electrons will reduce ferric ion into ferrous through carbon cloth and produce electricity.We recorded open circuit voltage curve and load voltage curve of MFCs in each different systems. Also, we have measured the biomass of each system in order to ensure whether the improved electricity could be attributed to more attached Shewanella cells on the anodes or the higher electroactivity of single cell.[11] </p> | + | <p>We have designed a bipolar chamber MFC this year. Proton exchange membrane divided it into anode chamber and cathode chamber. Anode chamber containing S.oneidensis, nutrient substance(LB、lactate ) or other electrical producing microbes were sealed to prevent the entry of external oxygen. Considering safety and oxidation-reduction potential, we put ferric chloride solution in cathode chamber so that S.oneidensis can transfer electrons outside of their membranes by electron transport chain. Then electrons will reduce ferric ion into ferrous through carbon cloth and produce electricity. |
| + | We recorded open circuit voltage curve and load voltage curve of MFCs in each different systems. Also, we have measured the biomass of each system in order to ensure whether the improved electricity could be attributed to more attached Shewanella cells on the anodes or the higher electroactivity of single cell.[11] </p> | ||
<div class="col-md-6 col-md-offset-2" style="text-align: center;"> | <div class="col-md-6 col-md-offset-2" style="text-align: center;"> | ||
| − | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/ | + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/2/2c/T--HUST-China--2018-description-pic1.png"> |
</div> | </div> | ||
| − | <div class="col-md-12"> | + | |
| − | < | + | <div class="col-md-12"> |
| + | <h3><b>Symbiosis<b/></h3> | ||
<p>Obviously, the ecological relationship between microorganisms is very complex. There is not only the competition between them for the nutrient, but also the regulation of metabolites among them including induction, transgenosis and synergistic metabolism. Besides, it has been found that the co-culture of microorganisms can improve the electric efficiency of Microbial Fuel Cell under certain conditions. </p> | <p>Obviously, the ecological relationship between microorganisms is very complex. There is not only the competition between them for the nutrient, but also the regulation of metabolites among them including induction, transgenosis and synergistic metabolism. Besides, it has been found that the co-culture of microorganisms can improve the electric efficiency of Microbial Fuel Cell under certain conditions. </p> | ||
| − | + | <p>By consulting literature, we found two kinds of microorganisms——Synechocystis sp. PCC 6803 and Rhodopseudomonas palustris, both of which can utilize light energy and provide lactate to S.oneidensis after doing molecular construction. </p> | |
| − | <p>By consulting literature, we found two kinds of microorganisms——Synechocystis and Rhodopseudomonas palustris, both of which can utilize light energy and provide lactate to S.oneidensis after doing molecular construction. </p> | + | |
<p>In order to provide a basic growth environment, we mix the culture medium of different strains.(Please refer to our protocol section for the composition of the mediums.)</p> | <p>In order to provide a basic growth environment, we mix the culture medium of different strains.(Please refer to our protocol section for the composition of the mediums.)</p> | ||
| Line 380: | Line 434: | ||
</div> | </div> | ||
| − | <div class="col-md-12"> | + | <div class="col-md-12"> |
| − | <p | + | |
| − | <p>Lactate produced by Synechocystis PCC6803 can be used as the optimal carbon source for Shewanella. At the same time, | + | <p><b>Synechocystis-Shewanella MFC <b/></p> |
| − | + | <p>Lactate produced by Synechocystis PCC6803 can be used as the optimal carbon source for Shewanella. At the same time, oxygen produced by Synechocystis would reduce the efficiency of Shewanella’s electricity production. However, it is proved by our electrogenesis experiment that engineered Synechocystis PCC6803 could function and improve the efficiency.[12]. </p> | |
| − | + | ||
| − | <p> | + | <div class="col-md-8" style="text-align: center;"> |
| − | + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/b/b0/T--HUST-China--2018-result-001.png"> | |
| − | + | </div> | |
| − | + | <div class="col-md-12" style="text-align: center;"> | |
| − | + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/7/71/T--HUST-China--2018-description-logo29.png.gif"> | |
| + | </div> | ||
| + | |||
| + | <div class="col-md-12"> | ||
| + | <p><b>Rhodopseudomonas-Shewanella MFC <b/></p> | ||
| + | <p>Rhodopseudomonas palustris is another partner we found to produce lactate and help Shewanella to produce electricity. Also, Rhodopseudomonas palustris would not produce oxygen which would reduce the efficiency of electricity production. Besides, acetate which is the metabolic waste of Shewanella could be utilized by Rhodopseudomonas palustris. Thus, we hypothesized that Rhodopseudomonas palustris have more potential as the partner of Shewanella. | ||
| + | </p> | ||
| + | </div> | ||
| − | + | ||
| − | + | ||
| − | + | </div> | |
| − | + | <div class="col-md-8" style="text-align: center;"> | |
| − | + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/0/07/T--HUST-China--2018-result-002.png"> | |
| + | </div> | ||
| + | <div class="col-md-12" style="text-align: center;"> | ||
| + | <img class="img-responsive" src="https://static.igem.org/mediawiki/2018/0/0a/T--HUST-China--2018-description-logo30.png.gif"> | ||
| + | </div> | ||
| − | + | </div> | |
| − | + | </div> | |
| + | </div> | ||
| + | <div class="row"> | ||
| + | <div class="col-md-12"> | ||
| − | <span> | + | <h3><strong>Part4: <span class="red-content">Future plan</span></strong></h3> |
| − | + | <div class="row"> | |
| + | <div class="col-md-12 info-blocks"> | ||
| + | <br/> | ||
| + | <p>4.1 Since the whole circuits of lactate utilization part and NADH production part are separated, we could not improve lactate utilization and NADH production at the same time. We would find a method to integrate these two circuits and get a full-function Shewanella, which has the electrogenesis efficiency to maximum extent.</p> | ||
| + | <br/> | ||
| + | <p>4.2 As Synechocystis PCC6803 and Rhodopseudomonas palustris are used to transfer light energy to lactate, we would like to replace the promoter with light inducible promoter, which would make the engineered bacteria more intelligent.</p> | ||
| + | <br/> | ||
| + | <p>4.3 Sewage is a type of waste water which contains many kinds of nutrients. We want to replace LB medium with sewage and make our device much more environmentally friendly.</p> | ||
| + | <br/> | ||
| + | <p>4.4 We also want to integrate the targeted genes into the genome of engineered bacteria so that we can not only use medium without antibiotics which would reduce the growth rate of bacteria, but also have no need to take the plasmid loss into consideration.</p> | ||
| + | </div> | ||
| − | |||
| − | |||
| − | < | + | </div> |
| − | + | </div> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | <span>[7] Transport of L-Lactate, D-Lactate, and Glycolate by the LldP and GlcA Membrane Carriers of Escherichia coli Volume 290, Issue 2, 18 January 2002, Pages 824-829 </span></p> | + | <h4><strong>Reference </strong></h4> |
| − | + | ||
| − | + | <p>[1]Henrike Niederholtmeyer, Bernd T. Wolfstädter, David F. Savage, Pamela A. Silver, Jeffrey C. Way. Engineering Synechocystis sp. PCC 6803 to Synthesize and Export Hydrophilic Products. Applied and Environmental Microbiology. 2010, 76(11): 3462-3466.</p> | |
| − | <span>[8] Enhancement of Hydrogen Production and Carbon Fixation in Purple Nonsulfur Bacterium Bacterium by Synthetic Biology Shou-Chen Lo</span></p> | + | <p><span>[2] |
| − | + | Research progress and application of Rhodopseudomonas palustris | |
| + | [Journal] Wang Yuexian, Liu Dehai - Henan Animal Husbandry and Veterinary Medicine (Comprehensive Edition) 2015, Issue 1</span></p> | ||
| + | <p><span>[3] ZHOU Maohong, ZHAO Xiaowei, WU Xuechang. Study on the ability of photosynthetic bacteria Rhodopseudomonas palustris to assimilate phosphorus[J]. Bulletin of Science and Technology, 2002(02): 142-146.</span></p> | ||
| + | <p><span>[4] KEGG, https://www.genome.jp/kegg/pathway.html</span></p> | ||
| + | <p><span>[5] Cloning and sequence analysis of the gene encoding Lactococcus lactis malolactic enzyme: relationships with malic enzymes FEMS Microbiol Lett. 1994 Feb 1;116(1):79-86 </span></p> | ||
| + | <p><span>[6] Fine tuning the transcription of ldhA for d-lactate production August 2012, Volume 39, Issue 8, pp 1209–1217 </span></p> | ||
| + | <p><span>[7] Transport of L-Lactate, D-Lactate, and Glycolate by the LldP and GlcA Membrane Carriers of Escherichia coli Volume 290, Issue 2, 18 January 2002, Pages 824-829 </span></p> | ||
| + | <p><span>[8] Enhancement of Hydrogen Production and Carbon Fixation in Purple Nonsulfur Bacterium Bacterium by Synthetic Biology Shou-Chen Lo</span></p> | ||
| + | <p><span>[9] Jcat, http://www.jcat.de/#opennewwindow </span></p> | ||
| + | <p><span>[10] Genomic reconstruction of | ||
| + | Shewanella oneidensis MR-1 metabolism reveals a previously uncharacterized machinery for lactate utilization PNAS February 24, 2009 106 (8) 2874-2879 </span></p> | ||
| + | <p><span>[11] Modular Engineering Intracellular NADH Regeneration Boosts Extracellular Electron Transfer of Shewanella oneidensis MR‑1 ACS Synth. Biol. 2018, 7, 885−895</span></p> | ||
| + | <p><span>[12]Varman A. M., Yu Y., You L. & Tang Y. J. Photoautotrophic production of d-lactate in an engineered cyanobacterium. Microb. Cell Fact. 12, 117 (2013). </span></p> | ||
| + | </div> | ||
| + | </div> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
</div> | </div> | ||
</section> | </section> | ||
| Line 477: | Line 549: | ||
</script> | </script> | ||
</body> | </body> | ||
| − | |||
Latest revision as of 03:41, 18 October 2018
Description
To convert optical energy into electric energy in a clean and sustainable way, Optopia is designed as a photovoltaic system consisting of two subsystems: photosynthetic microorganism system (Synechocystis sp. or Rhodopseudomonas palustris) and electrogenic microorganism system (Shewanella oneidensis). Synthetic biology strategies are applied to the system to trigger production and export of lactate in photosynthetic microorganisms, as well as to improve efficiency of lactate utilization and extracellular electron generation in electrogenic microorganism system.
Synechocystis, one kind of cyanobacteria, is more mature than Rhodopseudomonas in lactate production, but generating lots of oxygen during photosynthesis. Given the fact that Shewanella favors anaerobic environment for electricity production, Rhodopseudomonas may serve as a better carbon resource provider for Shewanella, not only because of its anaerobic photosynthesis maintaining an anaerobic environment required for extracellular electron generation in Shewanella, but also due to its capacity of reusing the waste from Shewanella. Hence, we construct a Synechocystis- Shewanella MFC and a Rhodopseudomonas - Shewanella MFC to find an optimized version of Optopia, maximzing the conversion of optical energy to electric energy.
Part1: Photosynthetic microorganism system

1.1 Synechocystis sp.
Synechocystis sp. PCC 6803 is often chosen as engineering microorganism to convert optical energy into chemical energy. In our project, we chose Synechocystis PCC6803 for optical energy conversion. Synechocystis sp. PCC 6803 produces lactate for the electricity production of Shewanella. However, Synechocystis sp. PCC 6803 itself lacks a pathway for producing lactate, and as a photoautotrophic microorganism, Synechocystis sp. PCC 6803 lacks a lactate transporter to transport lactate out of the cell[1]. Therefore, we have designed two strategies to modify Synechocystis sp. PCC 6803.

Strategy 1:
The lactate dehydrogenase gene and the lactate transporter gene are combined in one circuit to achieve lactate production and transportation. For lactate dehydrogenase gene, we chose ldhD, while ldhDc is a codon-optimized version of ldhD, ldhDnARSdR is ldhD with D176A/I177R/F178S/N180R, and ldhDARSdR is the codon-optimized version of ldhDnARSdR. These codon optimizations are aimed at increasing the production of lactate. The lldP protein gene is used to transport the lactate out of the cell. The lldP protein has 12 transmembrane alpha-helical segments and generally lacks cleaved signal sequences. Besides, it cotransports lactate with a proton[1]. In order to detect the expression of these two genes, we add the Flag and 6 × His sequences respectively after the two.

Figure 1. circuit of ldhD-lldP.

Figure 2. circuit of ldhDC-lldP.

Figure 3. circuit of ldhDnARSdR-lldP.

Figure 4. circuit of ldhDARSdR-lldP.
Strategy 2:
In response to the preference for NADPH in the metabolism of Synechocystis sp. PCC 6803, transhydrogenase gene is used to achieve the goal of producing more NADPH, and the glycerol dehydrogenase gene is used to increase the lactate production. For transhydrogenase gene, we use TH and gldA for glycerol dehydrogenase. The gene lldP is also used to transport the lactate out of the cell. And the same as strategy 1, the Flag and 6 × His sequences are used to detect the expression of the two genes.

Figure 5. circuit of TH-gldA-lldP.
After successfully constructing these gene circuits, the shuttle plasmid pCK306 is used to transform the genes into Synechocystis sp. PCC 6803, and the yellow fluorescent protein gene on the pCK306 plasmid is used to detect the transformation.

1.2 Rhodopseudomonas palustris
Rhodopseudomonas palustris is a kind of Purple Non-sulfur Bacteria. Under anaerobic conditions, Rhodopseudomonas palustris can utilize hydrogen, sodium thiosulfate, hydrogen sulfide as electron donors for photoautotrophic growth. Furthermore, it can also have heterotrophic growth under microaerobic to aerobic conditions [2]. In addition, Rhodopseudomonas palustris is widely used in sewage treatment because it is environmental friendly and accessible [3]. Since it is capable of maintaining an anaerobic environment and reusing the acetate generated by Shewanella, Rhodopseudomonas palustris may be a better carbon source provider for Shewanella compared with Synechocystis sp. PCC 6803. Therefore, we decided to modify Rhodopseudomonas palustris so that it could produce lactate under the anaerobic condition and export it out of the cell. According to KEGG database [4], the metabolic pathways related to lactate metabolism in Rhodopseudomonas palustris are as follows: .

To enhance the production of lactate in Rhodopseudomonas palustris, we plan to promote the conversion efficiency of pyruvate to D-lactate and malate to L-lactate. Therefore, we decide to transfer these two genes, mleS [5] and ldhA, [6] into Rhodopseudomonas palustris to generate more lactate intracelluarly. Considering the necessity of transporting lactate out of the cells, we also apply the gene lldP [7] encoding lactate permease. Since the order of these three genes in a polycistronic lead to different expression levels [8], we build a model to optimize the gene order to maximize the gene expression. Also, we use codon optimized tool Jcat [9] to optimize the codons of these genes.

mleS:malate dehydrogenase, the conversion of malic acid to L-lactate.
ldhA:fermentative D-lactate dehydrogenase, NAD-dependent, convert pyruvate to D-lactate.
lldP:L-lactate permease, the lactate is transported out of the cell.
To validate our modeling results, we continue to build three different gene circuits which can determine the highest lactate production efficiency of our total circuit:



Part2: Electrogenic microorganism system

Shewanella oneidensis
Shewanella oneidensis is becoming more and more popular these years as it could transfer electron to the electrode and generate electricity. This fascinating power mainly attributes to a number of conductive c-type cytochromes (c-Cyts) including OmcA-MtrCAB and CymA. CymA could conduct electrons to the MtrCAB complex. Then, the bacteria would use its extension or vesicles of the outer membrane and periplasm to transfer outer membrane c-type cytochromes.

The amount of electricity produced by Shewanella is closely related to the bacteria’s metabolism.
①. Glycolysis: Glycolysis is the metabolic pathway that converts glucose C6H12O6 into pyruvate. As glyceraldehyde-3-phosphate turns into 1,3-Bisphosphoglycerate, NADH is generated, which would be used to transfer electron.
②. TCA cycle: TCA cycle is a series of chemical reactions happened in mitochondrion. The reactions of the cycle are carried out by eight enzymes and three of them including malate dehydrogenase could help to produce NADH.
③. Pyruvate fermentation: Pyruvate fermentation is a common metabolic pathway in bacteria. Several steps of pyruvate fermentation could also produce NADH and the related enzymes are pyruvate formate-lyase, lactate dehydrogenase and formate dehydrogenase.

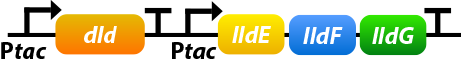
Shewanella oneidensis MR-1 prefers to use lactate as its carbon source since the amount of lactate-based biomass is more than acetate-based biomass or pyruvate-based biomass. Dld and lldEFG are D- and L-lactate dehydrogenase enzymes, which is the first step of utilizing lactate. To make the use of lactate more efficiently, we overexpress four genes: dld, lldE, lldF, lldG.[10]
①. dld: dld refers to FAD-dependent D-lactate dehydrogenase which could catalyze D-lactate’s transformation into pyruvate.
②. lldEFG: They could encode a L-lactate dehydrogenase complex which could catalyze D-lactate’s transformation into pyruvate.
To ensure that the genes would be expressed efficiently, we add a promoter before lldEFG:
NADH is a significant part of extracellular electron transfer(EET) as it could carry electron. Strenghthening the regeneration of NADH would make EET more efficiently.
To achieve this goal, we overexpress these four genes: gapA2, mdh, pflB, fdh. [11]
①. gapA: It encodes glyceraldehyde-3-phosphate dehydrogenase which could transform 3- phosphoglyceraldehyde into 1,3- diphosphoglycerate.
②. mdh: It encodes NAD dependent malate dehydrogenase which transforms malate into pyruvate
③. pflB: It encodes pyruvate formate-lyase to transform pyruvate into Acetyl-CoA.
④. fdh: It encodes formate dehydrogenase to transform formate into CO2..
Also, to ensure that the genes would be expressed efficiently, we add an promoter before pflB and fdh:

Part3: Whole design

Design of MFC(Microbial Fuel Cell)
We have designed a bipolar chamber MFC this year. Proton exchange membrane divided it into anode chamber and cathode chamber. Anode chamber containing S.oneidensis, nutrient substance(LB、lactate ) or other electrical producing microbes were sealed to prevent the entry of external oxygen. Considering safety and oxidation-reduction potential, we put ferric chloride solution in cathode chamber so that S.oneidensis can transfer electrons outside of their membranes by electron transport chain. Then electrons will reduce ferric ion into ferrous through carbon cloth and produce electricity. We recorded open circuit voltage curve and load voltage curve of MFCs in each different systems. Also, we have measured the biomass of each system in order to ensure whether the improved electricity could be attributed to more attached Shewanella cells on the anodes or the higher electroactivity of single cell.[11]

Symbiosis
Obviously, the ecological relationship between microorganisms is very complex. There is not only the competition between them for the nutrient, but also the regulation of metabolites among them including induction, transgenosis and synergistic metabolism. Besides, it has been found that the co-culture of microorganisms can improve the electric efficiency of Microbial Fuel Cell under certain conditions.
By consulting literature, we found two kinds of microorganisms——Synechocystis sp. PCC 6803 and Rhodopseudomonas palustris, both of which can utilize light energy and provide lactate to S.oneidensis after doing molecular construction.
In order to provide a basic growth environment, we mix the culture medium of different strains.(Please refer to our protocol section for the composition of the mediums.)
Synechocystis-Shewanella MFC
Lactate produced by Synechocystis PCC6803 can be used as the optimal carbon source for Shewanella. At the same time, oxygen produced by Synechocystis would reduce the efficiency of Shewanella’s electricity production. However, it is proved by our electrogenesis experiment that engineered Synechocystis PCC6803 could function and improve the efficiency.[12].


Rhodopseudomonas-Shewanella MFC
Rhodopseudomonas palustris is another partner we found to produce lactate and help Shewanella to produce electricity. Also, Rhodopseudomonas palustris would not produce oxygen which would reduce the efficiency of electricity production. Besides, acetate which is the metabolic waste of Shewanella could be utilized by Rhodopseudomonas palustris. Thus, we hypothesized that Rhodopseudomonas palustris have more potential as the partner of Shewanella.


Part4: Future plan
4.1 Since the whole circuits of lactate utilization part and NADH production part are separated, we could not improve lactate utilization and NADH production at the same time. We would find a method to integrate these two circuits and get a full-function Shewanella, which has the electrogenesis efficiency to maximum extent.
4.2 As Synechocystis PCC6803 and Rhodopseudomonas palustris are used to transfer light energy to lactate, we would like to replace the promoter with light inducible promoter, which would make the engineered bacteria more intelligent.
4.3 Sewage is a type of waste water which contains many kinds of nutrients. We want to replace LB medium with sewage and make our device much more environmentally friendly.
4.4 We also want to integrate the targeted genes into the genome of engineered bacteria so that we can not only use medium without antibiotics which would reduce the growth rate of bacteria, but also have no need to take the plasmid loss into consideration.
Reference
[1]Henrike Niederholtmeyer, Bernd T. Wolfstädter, David F. Savage, Pamela A. Silver, Jeffrey C. Way. Engineering Synechocystis sp. PCC 6803 to Synthesize and Export Hydrophilic Products. Applied and Environmental Microbiology. 2010, 76(11): 3462-3466.
[2] Research progress and application of Rhodopseudomonas palustris [Journal] Wang Yuexian, Liu Dehai - Henan Animal Husbandry and Veterinary Medicine (Comprehensive Edition) 2015, Issue 1
[3] ZHOU Maohong, ZHAO Xiaowei, WU Xuechang. Study on the ability of photosynthetic bacteria Rhodopseudomonas palustris to assimilate phosphorus[J]. Bulletin of Science and Technology, 2002(02): 142-146.
[4] KEGG, https://www.genome.jp/kegg/pathway.html
[5] Cloning and sequence analysis of the gene encoding Lactococcus lactis malolactic enzyme: relationships with malic enzymes FEMS Microbiol Lett. 1994 Feb 1;116(1):79-86
[6] Fine tuning the transcription of ldhA for d-lactate production August 2012, Volume 39, Issue 8, pp 1209–1217
[7] Transport of L-Lactate, D-Lactate, and Glycolate by the LldP and GlcA Membrane Carriers of Escherichia coli Volume 290, Issue 2, 18 January 2002, Pages 824-829
[8] Enhancement of Hydrogen Production and Carbon Fixation in Purple Nonsulfur Bacterium Bacterium by Synthetic Biology Shou-Chen Lo
[9] Jcat, http://www.jcat.de/#opennewwindow
[10] Genomic reconstruction of Shewanella oneidensis MR-1 metabolism reveals a previously uncharacterized machinery for lactate utilization PNAS February 24, 2009 106 (8) 2874-2879
[11] Modular Engineering Intracellular NADH Regeneration Boosts Extracellular Electron Transfer of Shewanella oneidensis MR‑1 ACS Synth. Biol. 2018, 7, 885−895
[12]Varman A. M., Yu Y., You L. & Tang Y. J. Photoautotrophic production of d-lactate in an engineered cyanobacterium. Microb. Cell Fact. 12, 117 (2013).



