| Line 1: | Line 1: | ||
<!--THIS COMES BEFORE THE CONTENT--> | <!--THIS COMES BEFORE THE CONTENT--> | ||
| − | {{Template:Toulouse-INSA-UPS/SCROLLSPY}} <html> | + | {{Template:Toulouse-INSA-UPS/SCROLLSPY}} |
| + | <html> | ||
<link rel="stylesheet" type="text/css" href="https://2018.igem.org/wiki/index.php?title=Template:Toulouse-INSA-UPS/BS-CSS&action=raw&ctype=text/css"/> | <link rel="stylesheet" type="text/css" href="https://2018.igem.org/wiki/index.php?title=Template:Toulouse-INSA-UPS/BS-CSS&action=raw&ctype=text/css"/> | ||
<link rel="stylesheet" type="text/css" href="https://2018.igem.org/wiki/index.php?title=Template:Toulouse-INSA-UPS/PERSO-CSS&action=raw&ctype=text/css"/> | <link rel="stylesheet" type="text/css" href="https://2018.igem.org/wiki/index.php?title=Template:Toulouse-INSA-UPS/PERSO-CSS&action=raw&ctype=text/css"/> | ||
<!--Banner--> | <!--Banner--> | ||
| − | + | <div class="container-fluid parallax" style="background-image: url('https://static.igem.org/mediawiki/2018/a/a4/T--Toulouse-INSA-UPS--project--Brice--Banner.jpg'); background-size: cover; background-position-y: center;" id="BANNER"></div> | |
</html> {{Template:Toulouse-INSA-UPS/MENU}} <html> | </html> {{Template:Toulouse-INSA-UPS/MENU}} <html> | ||
| − | + | ||
</div> | </div> | ||
</html> {{Template:Toulouse-INSA-UPS/CONTENT-BEGINNING}} <html> | </html> {{Template:Toulouse-INSA-UPS/CONTENT-BEGINNING}} <html> | ||
| + | |||
<!--CONTENT COMES HERE--> | <!--CONTENT COMES HERE--> | ||
| − | + | <!--Here goes the content--> | |
| − | + | <!--TITLE+INTRO--> | |
| − | + | <h1 class="heavy">RESULTS</h1> | |
| + | <hr/> | ||
| + | <p> | ||
| + | The whole Cerberus project is based on the construction and validation of a proteic platform to link very different molecules together. This platform is composed of three heads. The first head is a CBM3a domain which specifically bind cellulose. The second head is a streptavidin domain with a strong affinity to biotinylated compounds. The third head is based on the presence of an unnatural amino acid to covalently link any molecule with an alkyne moiety. | ||
| + | </p> | ||
| + | <p>To validate the Cerberus platform, we worked head by head, starting from the CBM3a validation, then the streptavidin domain assessment and finally the demonstration of the unnatural amino acid capacity to covalently bound alkynilated molecules. Thus, our first construction has only one head and was named Sirius, after the brightest star of the northern hemisphere alpha canis majoris. The second construction presents both the CBM3a and streptavidin heads and was nicknamed Orthos, as a two-headed mythological dog. For sure, the final construction is Cerberus and its three heads.</p> | ||
| − | + | <div id="HP_Tab_Group"> | |
| − | + | <!--SUB MENU OF results--> | |
| + | <ul class="nav nav-pills d-flex flex-row flex-wrap" role="tablist"> | ||
| + | <li class="nav-item"> | ||
| + | <a class="nav-link btn btn-secondary rounded-top corner-bottom mr-1 active" data-toggle="pill" href="#Results_Part1" role="tab">Sirius: CBM3a</a> | ||
| + | </li> | ||
| + | <li class="nav-item"> | ||
| + | <a class="nav-link btn btn-secondary rounded-top corner-bottom mr-1" data-toggle="pill" href="#Results_Part2" role="tab">Orthos: Streptavidin</a> | ||
| + | </li> | ||
| + | <li class="nav-item"> | ||
| + | <a class="nav-link btn btn-secondary rounded-top corner-bottom mr-1" data-toggle="pill" href="#Results_Part3" role="tab">Cerberus: AzF</a> | ||
| + | </li> | ||
| + | <li class="nav-item"> | ||
| + | <a class="nav-link btn btn-secondary rounded-top corner-bottom mr-1" data-toggle="pill" href="#Results_Part4" role="tab">Bacterial Cellulose</a> | ||
| + | </li> | ||
| + | </ul> | ||
| − | + | <!--Common Member banner--> | |
| − | + | <div class="hr_img"> | |
| − | + | <img class="hr_img filter-gray-90" src="https://static.igem.org/mediawiki/2018/1/11/T--Toulouse-INSA-UPS--bannersmall--Brice--Banner.jpg" alt="A banner" /> | |
| − | + | </div> | |
| − | + | ||
| − | + | <div class="tab-content"> | |
| − | + | ||
| − | + | <!--PART 1 : Sirius--> | |
| − | + | <div class="tab-pane fade show active" id="Results_Part1" role="tabpanel"> | |
| − | + | <h2 id="Sirius" class="heavy">Sirius: CBM3a</h2> | |
| − | + | <hr/> | |
| − | + | ||
| − | + | <h3 id="Background_Sirius" class="heavy">Background</h3> | |
| − | + | <p> | |
| − | + | Sirius is a fusion protein between CBM3a and mRFP1. This design, which consists in fusing CBM3a to a fluorescent moiety, allowed us to investigate the binding capability of the CBM3a domain to cellulose through the four aromatic amino acids of its binding domain. mRFP1 was chosen since it is both a coloured and fluorescent protein. | |
| − | + | </p> | |
| − | + | <h3 id="Key_Achievements_Sirius" class="heavy">Key Achievements</h3> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | <div class="tab-pane fade" id=" | + | <p> |
| − | + | <ul> | |
| + | <li> <p><i>Cloning of the part encoding Sirius</i></p></li> | ||
| + | <li> <p><i>Overexpression and purification of Sirius</i></p></li> | ||
| + | <li> <p><i>Fixation of Sirius to cellulose</i></p></li> | ||
| + | </ul> | ||
| + | </p> | ||
| + | <h3 id="Materials_Methods_Sirius" class="heavy">Materials and Methods</h3> | ||
| + | <p> | ||
| + | The fused CBM3a and mRFP1 sequences were cloned into the pET28 expression vector in fusion with a histidine tag (Figure 1). | ||
| + | </p> | ||
| + | <figure class="figure" style="text-align:center;"> | ||
| + | <img style="width : 70%; height: auto;" src="https://static.igem.org/mediawiki/2018/4/4f/T--Toulouse-INSA-UPS--Results--Youn--constructSirius.png" class="figure-img img-fluid rounded" alt="A generic square placeholder image with rounded corners in a figure."> | ||
| + | <figcaption class="figure-caption"><strong>Figure 1:</strong> scheme of the Sirius construction</figcaption> | ||
| + | </figure> | ||
| + | <p>The resulting construct was transformed into <em>E. coli</em> strain and expression of the recombinant protein was induced using IPTG. The His-tagged protein was then purified on IMAC resin charged with cobalt and used in cellulose pull down assays. For the experimental details, see (link).</p> | ||
| + | <h3 id="Results_Discussion_Sirius" class="heavy">Results and Discussion</h3> | ||
| + | <h4 id="Production_purification_Sirius" class="heavy">Production and purification of Sirius</h4> | ||
| + | <p>Interestingly, <i>E. coli</i> turned a deep pink shade after induction, indicating a strong production of Sirius (Figure 2).</p> | ||
| + | <div style="text-align:center"> | ||
| + | <figure class="figure" id="figure" style="text-align:center;"> | ||
| + | <img style="width : 40%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/9/96/T--Toulouse-INSA-UPS--Results--Youn--fig2sirius.jpg" class="figure-img img-fluid rounded" alt="SDS Page"> | ||
| + | <figcaption id="figcaption" class="figure-caption"><i><strong>Figure 2:</strong> E. coli expressing Sirius </i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <div style="text-align:center"> | ||
| + | <figure class="figure" id="figure" style="text-align:center;"> | ||
| + | <img style="width : 60%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/c/c3/T--Toulouse-INSA-UPS--Collaborations--GB--SDSPAGE_Analysis.jpg" class="figure-img img-fluid rounded" alt="SDS Page"> | ||
| + | <figcaption id="figcaption" class="figure-caption"><i><strong>Figure 3:</strong> SDS-PAGE analysis of Sirius purification. (CFE: cell free extract, FT: flow through, W: washes, E1/40: elution with 40mM imidazole, E1/100: elution with 100 mM imidazole, E2/100: elution with 100 mM imidazole, E1/300: elution with 300 mM imidazole, MW: molecular weight ladder.) </i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <p> | ||
| + | As shown in Figure 3, we successfully purified the Sirius protein. Indeed, induction with IPTG produced a large amount of a protein at the expected size for Sirius (52 kDa, lane CFE). This protein was then found predominant in elution samples (E1/40 and E1/100). We estimated the degree of purity of full-length Sirius at about 72%. In addition to the full-length protein, we observed several extra bands that likely correspond to proteolysis products. | ||
| + | </p> | ||
| + | |||
| + | |||
| + | |||
| + | <h4 id="Validation_Sirius" class="heavy">Validation</h4> | ||
| + | <p> | ||
| + | Once produced in <em<E. coli</Em>, Sirius binding to cellulose was tested using pull down assays. 70 μM of Sirius protein or mRFP1 (without CBM3a) or buffer were incubated with cellulose. After five washes with Tris-HCl, cellulose pellets were recovered and the associated fluorescence was measured.</p> | ||
| + | |||
| + | <p> | ||
| + | When mRFP1 was added alone to cellulose, its associated colour mostly remained in the supernatant (Figure 4). When Sirius was associated to cellulose, the pinkish colour was clearly associated to the cellulose pellet. Quantification of the cellulose-associated fluorescence backed up these observations (Figure 5): control experiments showed that only background levels of fluorescence are retained in the cellulose pellet incubated with mRFP1 alone. These experiments have been performed in triplicate and statistical analyses indicate that the high level of fluorescence obtained with Sirius is definitively significant. These results clearly show that the CBM3a domain of Sirius interacts with cellulose, and thus mediates binding of mRFP1 protein to cellulose. | ||
| + | </p> | ||
| + | <div style="text-align:center"> | ||
| + | <figure id="figure2" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 60%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/6/61/T--Toulouse-INSA-UPS--Results--Youn--CPDSirius.png" class="figure-img img-fluid rounded" alt="Fluorescence Retained"> | ||
| + | <figcaption id="figcaption2" class="figure-caption"><i><strong>Figure 4:</strong> Picture of cellulose pull-down assay with mRFP1 alone (left tube) or with the Sirius construction (right tube).</i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <div style="text-align:center"> | ||
| + | <figure id="figure2" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 60%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/b/bb/T--Toulouse-INSA-UPS--Collaborations--GB--FluoRetained.jpg" class="figure-img img-fluid rounded" alt="Fluorescence Retained"> | ||
| + | <figcaption id="figcaption2" class="figure-caption"><i><strong>Figure 5:</strong> Binding of Sirius to cellulose. Fluorescence retained in the cellulose pellet after pull down (triplicate test)</i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | <!--PART 2 : Orthos--> | ||
| + | <div class="tab-pane fade" id="Results_Part2" role="tabpanel"> | ||
| + | <h2 id="Orthos" class="heavy">Orthos: Streptavidin and biotinylated compounds</h2> | ||
| + | <hr/> | ||
| + | |||
| + | <h3 id="Background_Orthos" class="heavy">Background</h3> | ||
| + | <p> | ||
| + | Next step was to assess the binding capacity of the streptavidin linker. Orthos, named after the guardian of Geryon's cattle, is a fusion protein between CBM3a and a monomeric streptavidin head. The strong affinity of streptavidin for biotin (dissociation constant of 10<sup>-13</sup>M) will allow to tightly bind biotinylated organic molecules to Orthos. We used two types of biotinylated compounds to monitor the ability of our platform to functionalize cellulose: fluorophores (mTagBFP and fluorescein) and antimicrobial peptide (scygonadin). | ||
| + | </p> | ||
| + | |||
| + | <h3 id="Key_Achievements_Orthos" class="heavy">Key Achievements</h3> | ||
| + | |||
| + | <p> | ||
| + | <ul> | ||
| + | <li> <p><i>Cloning of the part encoding Orthos</i></p></li> | ||
| + | <li> <p><i>Production of Orthos carrying monomeric streptavidin</i></p></li> | ||
| + | <li> <p><i>Production of biotinylated fluorophores</i> in vitro <i>and</i> in vivo</p></li> | ||
| + | <li> <p><i>Functionalization of cellulose with biotinylated compounds</i></p></li> | ||
| + | </ul> | ||
| + | </p> | ||
| + | <h3 id="Materials_Methods_Orthos" class="heavy">Materials and Methods</h3> | ||
| + | <p> | ||
| + | The fusion between monomeric streptavidin and CBM3a was cloned into the pET28 expression vector. The resulting construct was transformed into E. coli strain BL21 and expression of the recombinant protein was induced using IPTG. For Orthos, the histidine tag present on pET28 will not be fused to the CBM3a domain since an amber Stop codon is present between them (Figure 1). The protein was therefore not purified on the IMAC column but purified on Regenerated Amorphous Cellulose (RAC) and used in cellulose pull down assays. For the experimental details, see our <a href="https://2018.igem.org/Team:Toulouse-INSA-UPS/Experiments">experiments page</a>. | ||
| + | </p> | ||
| + | <div style="text-align:center"> | ||
| + | <figure id="figure2" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 60%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/7/70/T--Toulouse-INSA-UPS--Results--Youn--constructorthos.png" class="figure-img img-fluid rounded" alt="Fluorescence Retained"> | ||
| + | <figcaption id="figcaption2" class="figure-caption"><i><strong>Figure 1:</strong> scheme of the Orthos construction </i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <h3 id="Results_Discussion_Orthos" class="heavy">Results and Discussion</h3> | ||
| + | <h4 id="Purification_Orthos" class="heavy">Purification of Orthos</h4> | ||
| + | |||
| + | |||
| + | <div style="text-align:center"> | ||
| + | <figure id="figure3" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 45%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/d/d9/T--Toulouse-INSA-UPS--Collaborations--GB--SDSPAGE_Analysis2.jpg" class="figure-img img-fluid rounded" alt="SDS-PAGE"> | ||
| + | <figcaption id="figcaption3" class="figure-caption"><i><strong>Figure 2:</strong> SDS-PAGE analysis of purification of Orthos (S: supernatant, W1, W2: washes, E: elution, MW: molecular weight ladder)</i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <p> | ||
| + | Figure 2 presents the results of the purification of Orthos on RAC. The supernatant (S) contained all proteins produced in <i>E. coli</i>, and washes W1 and W2 allowed to eliminate most of non-specific interactions with cellulose. The elution step, using ethylene glycol, released the Orthos protein from RAC. The band at about 40 kDa corresponds to Orthos (expected size: 39 kDa) and the band at 15 kDa likely corresponds to a proteolysis fragment of Orthos containing only the CBM3a module. </p> | ||
| + | <p> | ||
| + | We estimated that the purification levels of the monomeric Orthos was about 45%, which is sufficient for our validation assays. | ||
| + | </p> | ||
| − | + | <h4 id="Validation_Orthos" class="heavy">Validation of monomeric Orthos</h4> | |
| − | + | <h5 id="Validation_Invivo_Orthos" class="heavy">Validation using<i> in vivo </i>biotinylation</h5> | |
| − | + | <p> | |
| − | + | In a first set of experiments, we assessed the ability of Orthos to functionalize cellulose with a compound biotinylated <i>in vivo</i>. For that purpose, we took advantage of a novel system for in vivo protein biotinylation using the biotin ligase BirA. We thus co-expressed BirA and a version of the Blue Fluorescent Protein (BFP) fused to an Avitag in <i>E. coli</i>. The Avitag sequence is recognized by BirA for biotin ligation. We added biotin in the culture medium during IPTG induction, in order to produce and purify biotinylated BFP.</p> | |
| − | + | <p>We then incubated 3.2 μM of Orthos with 32 μM of in vivo biotinylated BFP (Figure 3). For the control experiment BFP (without Orthos), the same quantity of BFP was added to have a relevant comparison. These samples were then incubated with cellulose. After three washes with Tris-HCl, fluorescence was measured in the cellulose pellets. As shown in Figure 3, fluorescence is twice more intense in Orthos sample than in the control sample containing BFP alone. These experiments have been performed in quadruplicate and a Mann Whitney statistical test indicated that the increased fluorescence signal obtained with Orthos is significant.</p> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | <div style="text-align:center"> | |
| − | + | <figure id="figure4" class="figure" style="text-align:center;"> | |
| − | + | <img style="width : 50%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/0/04/T--Toulouse-INSA-UPS--Collaborations--GB--FluoRetainedOrthos.jpg" class="figure-img img-fluid rounded" alt="Fluorescence Retained"> | |
| − | + | <figcaption id="figcaption4" class="figure-caption"><i><strong>Figure 3:</strong> Functionalization of cellulose with Orthos bound to biotinylated BFP. Fluorescence remaining on cellulose fraction after several washes (*Mann Whitney test p-value 0.01)</i></figcaption> | |
| − | + | </figure> | |
| − | + | </div> | |
| − | + | ||
| − | + | <p> | |
| − | + | These results demonstrate that Orthos binds efficiently to an in vivo biotinylated compound. In addition, they show that Orthos binds to cellulose and thus provides a proof of principle that Orthos design allows functionalization of cellulose with a fluorophore through its streptavidin linker. | |
| − | + | </p> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | <h5 id="Validation_Invitro_Orthos" class="heavy">Validation using <i>in vitro</i> chemical biotinylation</h5> | |
| − | + | <p> | |
| − | + | In a second set of experiments, we tested the ability of Orthos to functionalize cellulose with in vitro biotinylated compounds. Since we had both an azide-functionalized fluorescein (FITC: fluorescein isothiocyanate) and biotin-DBCO (an alkyne moiety), we imagined to combine both to produce biotinylated FITC. Besides, it was a nice way to challenge this click chemistry technology. We used the newly emerging technique Cu(I)-free strain-promoted alkyne-azide click chemistry (SPAAC) allowing to couple <i>in vitro </i>a molecule containing a dibenzocyclooctyne (DBCO) moiety to another molecule bearing an azide function. We used this technique to ligate <i>in vitro </i>biotin-DBCO to an azide-functionalized fluorescein (FITC), thus leading to the expected biotinylated FITC. | |
| − | + | </p> | |
| − | + | ||
| − | + | <div style="text-align:center"> | |
| − | + | <figure id="figure5" class="figure" style="text-align:center;"> | |
| − | + | <img style="width : 45%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/5/52/T--Toulouse-INSA-UPS--Collaborations--angeline--FluoRetainedOrthos2.jpg" class="figure-img img-fluid rounded" alt="Fluorescence Retained"> | |
| − | + | <figcaption id="figcaption5" class="figure-caption"><i><strong>Figure 4:</strong> Functionalization of cellulose with Orthos bound to in vitro-biotinylated FITC. Fluorescence remaining on cellulose pellet fraction after several washes (quadruplicate test). *Mann Whitney test p-value 0.1</i></figcaption> | |
| − | + | </figure> | |
| − | + | </div> | |
| − | + | ||
| + | <p> | ||
| + | Then, 58.0 μM of biotinylated FITC was incubated with 5.8 µM of Orthos protein. The same amount of FITC alone (58.0 μM) was used for the control experiment. These samples were incubated with cellulose and after five washes with Tris-HCl, fluorescence levels were measured in the cellulose pellet fractions (Figure 4). In the presence of Orthos, fluorescence in the cellulose pellet was about twice higher than in control experiments corresponding to the cellulose pellet incubated with FITC alone (without Orthos) or with the reaction buffer only. These experiments were performed in quadruplicate and a Mann Whitney statistical test indicated that the increased fluorescence signal obtained with Orthos is very significant. This result clearly shows that purified Orthos retains the ability to mediate the interaction between cellulose and a compound biotinylated <i>in vitro</i>. In addition, this result provides another strong evidence that Orthos allows to functionalize cellulose using a fluorescent molecule through its streptavidin linker. It also shows that we succeeded in creating biotinylated FITC by click chemistry.</p> | ||
| + | |||
| + | <h5 id="Validation_Scygonadin_Orthos" class="heavy">Validation using scygonadin</h5> | ||
| + | <p> | ||
| + | In order to confirm that the streptavidin moiety of Orthos is able to interact with different kind of biotinylated compounds, we also tried to bind it to an antimicrobial peptide, the scygonadin. To biotinylate this compound, we used the in vivo protein biotinylation system as the one used for BFP molecule. We co-expressed BirA with a version of scygonadin fused to the Avitag in <i>Pichia pastoris</i>. | ||
| + | </p> | ||
| − | + | <p> | |
| + | We then incubated Orthos with in vivo biotinylated scygonadin, and few hours later, we added cellulose (under form of a cellulose spot). After two washes with Tris-HCl, cellulose spot with Orthos-scygonadin were deposed on Petri dish pre-inoculated with a <i>E. coli </i>layer. We observed after incubation overnight small halos of inhibition for some samples (Figure 5). </p> | ||
| + | <div style="text-align:center"> | ||
| + | <figure id="figure6" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 55%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/5/55/T--Toulouse-INSA-UPS--Collaborations--angeline--Halo.jpg" class="figure-img img-fluid rounded" alt="Inhibition halo"> | ||
| + | <figcaption id="figcaption6" class="figure-caption"><i><strong>Figure 5:</strong> Halo of inhibition of Orthos alone ( C), scygonadin alone (S) and Orthos+scygonadin (C+S) samples</i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <h3 id="Perspectives_Orthos" class="heavy">Perspectives</h3> | ||
| + | <p> | ||
| + | We noticed that the inhibition halo was more important for the Orthos-scygonadin sample than the scygonadin alone control. Unfortunately, we repeated this test, but we did not obtain the same result. Therefore, we need to improve our experiment to prove the coupling between Orthos and biotinylated scygonadin. These results are nevertheless encouraging in the perspective of functionalizing cellulose using Orthos bound to antibiotic protein. | ||
| + | </p> | ||
| + | <h3 id="Perspectives_Orthos" class="heavy">Perspectives</h3> | ||
| + | <hr/> | ||
| + | <p>Our experiments using different kinds of biotinylated compounds provide convincing proofs of concept that Orthos can be conjugated, via its streptavidin head, to biotinylated organic molecules. The resulting molecules interact with cellulose and are therefore potent cellulose functionalizing compounds. </p> | ||
| + | <p>We can now envision to exploit the streptavidin linker of Orthos to ligate various compounds that can be biotinylated chemically (<i>in vitro</i>) or <i>in vivo</i>, via BirA co-expression.</p> | ||
| + | <p>In addition, we incorporated a TEV protease cleavage between the <i>N</i>-terminus linker and the streptavidin. This will allow to produce Orthos-bound biotinylated compounds that can be released by TEV cleavage if required, as in the case of odorant molecules for examples for a commercial use. </p> | ||
| + | </div> | ||
| + | |||
| + | <!--PART 3 : cerberus--> | ||
| + | <div class="tab-pane fade" id="Results_Part3" role="tabpanel"> | ||
| + | <h2 id="Cerberus" class="heavy">Cerberus: AzF</h2> | ||
| + | <hr/> | ||
| + | |||
| + | <h3 id="Background_Cerberus" class="heavy">Background</h3> | ||
| + | <p> | ||
| + | Cerberus, named after the guardian of the gates of the Underworld, is our three-headed protein made of the cellulose-binding molecular platform (CBM3a) fused at the N-terminus to the biotinylated molecule-binding head (monomeric streptavidin) and at the C-terminus to the unnatural amino acid azido-L-phenylalanine (AzF). The incorporation of AzF is achieved through the recognition of the amber stop codon at the C-terminus of CBM3a by an orthogonal AzF-charged tRNA. | ||
| + | </p> | ||
| + | |||
| + | <figure id="figure6" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 55%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/8/85/T--Toulouse-INSA-UPS--Results--Youn--constructcerberus.png" class="figure-img img-fluid rounded" alt="Inhibition halo"> | ||
| + | <figcaption id="figcaption6" class="figure-caption"><i><strong>Figure 1:</strong> Scheme of the Cerberus construction </i></figcaption> | ||
| + | </figure> | ||
| + | <p> | ||
| + | Coupling of molecules to the AzF head can be performed by click chemistry, either through SPAAC or through Cu(I)-catalyzed azide-alkyne click chemistry (CuAAC). To prove the versatility of the system, different compounds such as fluorescent proteins or paramagnetic beads have been clicked to Cerberus and thus associated to cellulose. | ||
| + | </p> | ||
| + | |||
| + | <h3 id="Key_Achievements_Cerberus" class="heavy">Key Achievements</h3> | ||
| + | <p> | ||
| + | <ul> | ||
| + | <li> <p><i>Cloning of the part encoding Cerberus</i></p></li> | ||
| + | <li> <p><i>Production of Cerberus</i></p></li> | ||
| + | <li> <p><i>Functionalization of cellulose</i></p></li> | ||
| + | </ul> | ||
| + | </p> | ||
| + | |||
| + | <h3 id="Materials_Methods_Cerberus" class="heavy">Materials and Methods</h3> | ||
| + | <p> | ||
| + | The fusion between the streptavidin linker and the CBM3a domain sequences followed by the amber stop codon was cloned into the pET28 expression vector in fusion with the His-tag. The resulting construct was co-transformed into E. coli strain with the pEVOL expression vector. The latter allows to express the amino acyl tRNA synthetase aaRS. In presence of AzF, this enzyme synthesizes the orthogonal AzF-tRNA for in vivo AzF incorporation in place of amber Stop codon. The Cerberus platform was produced by adding IPTG (Cerberus induction), L-arabinose (amino acyl tRNA synthetase production), and AzF to the medium. The His-tagged protein was then purified on IMAC resin charged with cobalt and used in cellulose pull down assays. For the experimental details, see <a href="https://2018.igem.org/Team:Toulouse-INSA-UPS/Experiments">experiments page</a>. | ||
| + | </p> | ||
| + | <h3 id="Results_Discussion_Cerberus" class="heavy">Results and Discussion</h3> | ||
| + | <h4 id="Purification_Cerberus" class="heavy">Production and purification of Cerberus</h4> | ||
| + | <p> | ||
| + | Cerberus production requires a deferred induction. We first induced the synthesis of aarS/tRNA pair with L-arabinose, and added AzF at the same time. One hour later, we induced the production of Cerberus with IPTG. | ||
| + | </p> | ||
| + | |||
| + | <div style="text-align:center"> | ||
| + | <figure id="figure7" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 28%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/7/70/T--Toulouse-INSA-UPS--Collaborations--angeline--SDSPAGE_cerberus.jpg" class="figure-img img-fluid rounded" alt="SDS-PAGE"> | ||
| + | <figcaption id="figcaption7" class="figure-caption"><i><strong>Figure 2:</strong> SDS-PAGE analysis of the production of Cerberus with monomeric streptavidin (NI: non-induced, I-AzF: induced without AzF, I+AzF: induced with AzF, E1: elution with 40 mM imidazole, E2: elution with 100 mM imidazole, E3: elution with 100 mM imidazole, MW: molecular weight ladder)</i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | |||
| + | <p> | ||
| + | Cerberus production and purification were analysed on SDS gel (Figure 2). Induction in the presence of AzF (Figure 2, lane I+AzF) led to the expression of two major bands at 37 and 39 kDa approximately compared to control conditions, namely the non-induced (lane NI) and induced without AzF (lane I-AzF). We hypothesized that the upper band would correspond to Cerberus (expected molecular weight of Cerberus with monomeric streptavidin: 41 kDa) and the lower bands to the Orthos proteins (Cerberus without the AzF and the His-tag). Surprisingly, both bands were present in the elution fractions which is incoherent since Orthos lacks an His-tag and was supposed to be washed out during purification steps. To confirm our hypothesis, we analysed the same fractions by western blot using antibodies detecting the His-tag (Figure 3). | ||
| + | </p> | ||
| + | |||
| + | <div style="text-align:center"> | ||
| + | <figure id="figure8" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 35%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/0/01/T--Toulouse-INSA-UPS--Collaborations--angeline--SDSPAGE_cerberus2.jpg" class="figure-img img-fluid rounded" alt="Western blot"> | ||
| + | <figcaption id="figcaption8" class="figure-caption"><i><strong>Figure 8:</strong> Western blot analysis of Cerberus production (NI: non-induced, I-AzF: induced without AzF, I+AzF: induced with AzF, E: elution, MW: molecular weight ladder)</i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | |||
| + | <p> | ||
| + | Anti-His-tag antibodies revealed a band at about 45 kDa in the sample corresponding to IPTG induction in the presence of AzF (Figure 3, lane I+AzF). This band is not present in control samples (Figure 3, lane NI and I-AzF), indicating that it corresponds to the Cerberus protein (theoretical size: 41 kDa). In addition to the full length protein, we observed several extra bands which very likely correspond to proteolysis products since they are detected with the anti-His-tag antibodies. Moreover, the band at 45 kDa is clearly detected in elution samples (Figure 3, lanes E). The band at 37 kDa observed on the SDS PAGE (Figure 2) is not observed on the Western blot. These results show that the experimental setup to produce Cerberus is also likely leading to the production of Orthos when the amber stop codon is not recognized by the AzF-charged orthogonal tRNA. Although Orthos does not contain a His-tag at its C-terminus, the protein seems to be efficiently co-purified with Cerberus. The basis of this observation is unclear but it may suggest that Orthos and Cerberus interact with each other, via their CBM3 or streptavidin moieties. | ||
| + | </p> | ||
| + | <p>We estimated that the purification level of Cerberus with monomeric streptavidin in the elution samples was about 62%. These data show that Cerberus was efficiently purified and can be used for pull down assays. </p> | ||
| + | |||
| + | <h4 id="Validation_Cerberus" class="heavy">Validation</h4> | ||
| + | <h5 id="Validation_Invivo_Cerberus" class="heavy">Validation using FITC (Fluorescein isothiocyanate) molecules</h5> | ||
| + | |||
| + | <div style="text-align:center"> | ||
| + | <figure id="figure7" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 50%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/f/f8/T--Toulouse-INSA-UPS--Collaborations--angeline--FluoRetainedCerberus.jpg" class="figure-img img-fluid rounded" alt="Fluorescence Retained"> | ||
| + | <figcaption id="figcaption7" class="figure-caption"><i><strong>Figure 4:</strong> Functionalization of cellulose with FITC conjugated to Cerberus. Fluorescence remaining in cellulose fraction after several washes (quadruplicate test). *Mann Whitney test p-value 0.03</i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <p> | ||
| + | To test the potential of Cerberus in functionalizing cellulose, we monitored its ability to mediate an interaction between cellulose and a fluorescent compound. To generate a fluorescently labelled Cerberus protein, we performed a SPAAC reaction on 3.2 µM Cerberus protein using 31.9 µM µg of FITC-DBCO. In control experiment, 31.9 µM of FITC alone was used. These samples were then incubated with cellulose and after five washes with Tris-HCl, fluorescence levels were measured in the cellulose pellet fractions (Figure 9). We observed that the fluorescence levels in the cellulose pellet incubated with Cerberus protein clicked to FITC were about 3 times higher than in the control experiments corresponding to the cellulose pellet incubated with FITC alone. These results show that Cerberus can efficiently be conjugated to fluorescent molecules bearing a DBCO group by click chemistry and that the resulting fluorescent molecules strongly interact with cellulose. Therefore, Cerberus is both a convenient and potent platform to functionalize cellulose. | ||
| + | </p> | ||
| + | |||
| + | |||
| + | <h5 id="Validation_Invitro_Cerberus" class="heavy">Validation using paramagnetic beads</h5> | ||
| + | <p> | ||
| + | We further characterize the functionality of Cerberus in a second set of experiments using paramagnetic beads. To bind paramagnetic beads to Cerberus, we performed a click reaction using 3.2 µM of Cerberus protein and 32 µM DBCO-conjugated paramagnetic beads. As a control experiment, 32 µM of paramagnetic beads were used. These samples were then incubated with cellulose, and after five washes with Tris-HCl, we measured the magnetisation of cellulose using a magnet. | ||
| + | </p> | ||
| + | <div style="text-align:center"> | ||
| + | <figure id="video1" class="figure" style="text-align:center;"> | ||
| + | <video controls preload="auto" muted="true" style="width : 50%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/1/14/T--Toulouse-INSA-UPS--Collaborations--angeline--ferocell.MOV" alt="Magnetic cellulose"> | ||
| + | |||
| + | </video> | ||
| + | <figcaption id="vidcaption" class="figure-caption"><i>video of the Cerberus-functionalized paramagnetic cellulose: on the left, control with paramagnetic beads alone; on the right, Cerberus-paramagnetic beads.</i></figcaption> | ||
| − | + | </figure> | |
| − | + | </div> | |
| − | + | <p> | |
| − | + | As observed on the video (Figure 5), the cellulose incubated with the Cerberus protein conjugated to paramagnetic beads is quickly and totally attracted by the magnet. In contrast, in the control experiment, no clear movement towards the magnet was observed. | |
| − | + | </p> | |
| − | + | ||
| + | <p> | ||
| + | These results show that Cerberus has the ability to interact simultaneously with cellulose and molecules with a DBCO group, confirming that the Cerberus platform allows to functionalize cellulose through its linker containing unnatural amino acid. | ||
| + | </p> | ||
| + | |||
| + | <h4 id="Validation_Scygonadin_Cerberus" class="heavy">Compounds functionalization</h4> | ||
| + | <p> | ||
| + | To go further in our project, we decided to functionalise two inorganic molecules, graphene and carbon nanotubes (CNT), using a reaction of diazotization. This experiment was performed with the help of the ENSIACET laboratory in Toulouse. To check functionalization, we analysed samples by Thermogravimetric Analysis (TGA). The concept of TGA consists in measuring the mass change of a sample depending on temperature and time, in a controlled atmosphere. We chose to perform the experiment under azote, an inert gas that avoids mass loss by oxidation, and we studied the mass change from 25 to 1,000 °C (15 °C/min). | ||
| + | </p> | ||
| + | |||
| + | <!-- <div style="text-align:left; background-color:yellow"> --> | ||
| + | <figure id="figure10" class="figure" style=" width:50%; float:left; text-align:center;"> | ||
| + | <img style="width:100%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/e/ed/T--Toulouse-INSA-UPS--Collaborations--angeline--massloss1.jpg" class="figure-img img-fluid rounded" alt="Mass loss"> | ||
| + | <figcaption id="figcaption10" class="figure-caption"><i><strong>Figure 6:</strong> Analysis of mass loss for functionalized CNT sample</i></figcaption> | ||
| + | </figure> | ||
| + | <!-- </div> --> | ||
| + | |||
| + | <!-- <div style="text-align:right"> --> | ||
| + | <figure id="figure11" class="figure" style=" width:50%; float:left; text-align:center;"> | ||
| + | <img style="width : 100%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/c/cb/T--Toulouse-INSA-UPS--Collaborations--angeline--massloss2.jpg" class="figure-img img-fluid rounded" alt="Mass loss"> | ||
| + | <figcaption id="figcaption11" class="figure-caption"><i><strong>Figure 7:</strong> Analysis of mass loss for non-functionalized CNT sample</i></figcaption> | ||
| + | </figure> | ||
| + | <!-- </div> --> | ||
| + | |||
| + | <p> | ||
| + | Figures 6 and 7 present the results of mass loss for non-functionalized Carbon Nanotube (CNT) and functionalized CNT samples, respectively. For functionalized CNT sample (Figure 7), we observed a mass loss of 27.4% around 100 °C corresponding to water loss. A second step of mass loss is observed around 550 °C, with a diminution of 19.8%. For the non-functionalized sample (Figure 6), we observed two mass losses at 150 and 750 °C corresponding to 1.5% and 3% of mass loss respectively. The significative difference between these two samples allowed us to conclude that the CNT functionalization has been successful. | ||
| + | </p> | ||
| + | |||
| + | <!-- <div style="text-align:center"> --> | ||
| + | <figure id="figure12" class="figure" style="width:50%; float:left; text-align:center;"> | ||
| + | <img style="width :100%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/1/1e/T--Toulouse-INSA-UPS--Collaborations--angeline--massloss3.jpg" class="figure-img img-fluid rounded" alt="Mass loss"> | ||
| + | <figcaption id="figcaption12" class="figure-caption"><i><strong>Figure 8:</strong> Analysis of mass loss for functionalized graphene sample</i></figcaption> | ||
| + | </figure> | ||
| + | <!-- </div> --> | ||
| + | |||
| + | <!-- <div style="text-align:center"> --> | ||
| + | <figure id="figure13" class="figure" style="width:50%; float:right; text-align:center;"> | ||
| + | <img style="width : 100%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/4/40/T--Toulouse-INSA-UPS--Collaborations--angeline--massloss4.jpg" class="figure-img img-fluid rounded" alt="Mass loss"> | ||
| + | <figcaption id="figcaption13" class="figure-caption"><i><strong>Figure 9:</strong> Analysis of mass loss for non-functionalized graphene sample</i></figcaption> | ||
| + | </figure> | ||
| + | <!-- </div> --> | ||
| + | |||
| + | <p> | ||
| + | Figures 8 and 9 present the results of mass loss for non-functionalized graphene and functionalized graphene samples respectively. For functionalized graphene sample (Figure 9), we observed a mass loss of 31.0% around 100 °C corresponding to water loss. A second step of mass loss is observed around 500 °C, with a diminution of 19.0%. For the non-functionalized sample (Figure 8), we did not observe a significative mass loss. So we can conclude that the graphene functionalization has been successful. | ||
| + | </p> | ||
| + | <p> | ||
| + | Our initial plan was to functionalize cellulose with CNT and graphene in order to change cellulose fibre rigidity and create conductive cellulose, respectively. Unfortunately, we did not have enough time during the course of the iGEM competition to complete these tests. Given the success of the other functionalization, we have reasonable hopes that these experiments will be successful too. | ||
| + | </p> | ||
| + | |||
| + | <h3 id="Perspectives_Cerberus" class="heavy">Perspectives</h3> | ||
| + | <p> | ||
| + | Our experiments provide robust proofs of concept that Cerberus can be conjugated, via its unnatural amino acid AzF, to organic or inorganic molecules bearing a DBCO group by click chemistry. The resulting molecules strongly interact with cellulose and are therefore potent cellulose functionalizing compounds. | ||
| + | </p> | ||
| + | <p> | ||
| + | We can now consider fixation of various compounds that can be chemically functionalized. We can also imagine double fixations, for example a fluorophore on the streptavidin head and paramagnetic beads on the AzF linker. This double fixation allows to envision endless possibilities to functionalize cellulose (see <a href="https://2018.igem.org/Team:Toulouse-INSA-UPS/Demonstrate">Demonstrate</a>). | ||
| + | </p> | ||
| + | </div> | ||
| + | <!--PART 4 : cellulose--> | ||
| + | <div class="tab-pane fade" id="Results_Part4" role="tabpanel"> | ||
| + | <h2 id="Bacterial Cellulose" class="heavy">Bacterial Cellulose production and functionalization</h2> | ||
| + | <hr/> | ||
| + | |||
| + | <h3 id="Background_Cellulose" class="heavy">Background</h3> | ||
| + | <p> | ||
| + | Our ultimate dream was to be able to produce functionalized cellulose in vivo. Some microorganisms naturally produce cellulose. One of these, <i>Gluconacetobacter hansenii</i>, can produce cellulose as a biofilm to protect itself. We took advantage of the work of iGEM Imperial team 2014 who has set up culture conditions to optimize cellulose production. Our challenge was to assess if our technology is compatible with bacterial cellulose as a first step before producing functionalized cellulose <i>in vivo</i>. | ||
| + | </p> | ||
| + | |||
| + | <h3 id="Key_Achievements_Cellulose" class="heavy">Key Achievements</h3> | ||
| + | <p> | ||
| + | <ul> | ||
| + | <li> <p><i>Production of about 100g of dried bacterial cellulose</i></p></li> | ||
| + | <li> <p><i>Functionalization of bacterial cellulose with Sirius</i></p></li> | ||
| + | </ul> | ||
| + | </p> | ||
| + | |||
| + | <h3 id="Materials_Methods_Cellulose" class="heavy">Materials and Methods</h3> | ||
| + | <p> | ||
| + | <i>G. hansenii </i>was grown in Hestrin-Shramm (HS) medium at 30 °C. After one week of culture, bacterial cellulose was extracted and purified. For the experimental details, see our <a href="https://2018.igem.org/Team:Toulouse-INSA-UPS/Experiments">experiments page</a>. | ||
| + | </p> | ||
| + | <p> | ||
| + | For the functionalization, bacterial cellulose was incubated with purified Sirius (CBM3a-mRFP1) or with purified mRFP1 alone as a control. Cellulose was then washed about 10 times with Tris-HCl buffer to remove non-attached proteins. | ||
| + | </p> | ||
| + | |||
| + | <h3 id="Results_Discussion_Cellulose" class="heavy">Results and Discussion</h3> | ||
| + | <div style="text-align:center"> | ||
| + | <figure id="figure14" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 50%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/b/b7/T--Toulouse-INSA-UPS--Collaborations--angeline--cellcolor.jpg" class="figure-img img-fluid rounded" alt="Fluorescence Retained"> | ||
| + | <figcaption id="figcaption14" class="figure-caption"><i><strong>Figure 1:</strong> Picture of functionalized cellulose with Sirius (CBM3a fused with mRFP1). Left: control mRFP1 alone; Right: Sirius</i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <div style="text-align:center"> | ||
| + | <figure id="figure14" class="figure" style="text-align:center;"> | ||
| + | <img style="width : 50%; heigth = auto;" src="https://static.igem.org/mediawiki/2018/e/ef/T--Toulouse-INSA-UPS--Results--Youn--uvbactcell.png" class="figure-img img-fluid rounded" alt="Fluorescence Retained"> | ||
| + | <figcaption id="figcaption14" class="figure-caption"><i><strong>Figure 2:</strong> Picture under UV of functionalized cellulose with Sirius (CBM3a fused with mRFP1). Left: control mRFP1 alone; Right: Sirius </i></figcaption> | ||
| + | </figure> | ||
| + | </div> | ||
| + | <p> | ||
| + | Figure 1 presents the results of the functionalization of our bacterial cellulose with the Sirius fluorescent protein. Only the cellulose sample incubated with Sirius kept the color after several washes with resuspension buffer. Likewise fluorescence remained associated to cellulose only for Sirius (Figure 2). These results show that CBM3a can interact with the bacterial cellulose and allows fixation of the mRFP1 protein to cellulose. | ||
| + | </p> | ||
| + | <h3 id="Perspectives_Cellulose" class="heavy">Perspectives</h3> | ||
| + | <p> | ||
| + | Now that we proved the capacity of CBM3a to interact with bacterial cellulose, we are confident that Orthos and Cerberus proteins should also interact with bacterial cellulose. Now the next step will be to produce <i>in vivo </i>functionalized bacterial cellulose. For that, we had planified to produce the Cerberus platform in the yeast <i>Pichia</i> pastoris because of its secretion capacity. Indeed, our idea was to co-culture both strain in the same reactor in order to produce functionalized cellulose <i>in vivo</i>. This work on P. pastoris was performed in the same time than our effort in E. coli but has not been as successful. We managed to produce the Orthos protein in the yeast but we never succeeded in producing Sirius and we never got the plasmid mendatory to produce the tRNA synthetase necessary for AzF incorporation in <i>P. pastoris</i>. Beside, the production of Orthos in the yeast was very low and not sufficient to conduct our assays. So production of functionalized <i>in vivo</i> will require to improve the <i>P. pastoris </i>part of our project. | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | </div> | ||
| − | |||
<!--CONTENT ENDS HERE--> | <!--CONTENT ENDS HERE--> | ||
</div> </html> {{Template:Toulouse-INSA-UPS/CONTENT-END}} <html> | </div> </html> {{Template:Toulouse-INSA-UPS/CONTENT-END}} <html> | ||
| Line 170: | Line 432: | ||
<li class="nav-item"> | <li class="nav-item"> | ||
<!--To previous page--> | <!--To previous page--> | ||
| − | <a class="nav-link ico" href="https://2018.igem.org/Team:Toulouse-INSA-UPS/ | + | <a class="nav-link ico" href="https://2018.igem.org/Team:Toulouse-INSA-UPS/Model"> |
<img class="ico" src="https://static.igem.org/mediawiki/2018/d/db/T--Toulouse-INSA-UPS--All--Yohann--Left_Arrow.png" alt="Left Arrow"/> | <img class="ico" src="https://static.igem.org/mediawiki/2018/d/db/T--Toulouse-INSA-UPS--All--Yohann--Left_Arrow.png" alt="Left Arrow"/> | ||
</a> | </a> | ||
| Line 182: | Line 444: | ||
<li class="nav-item"> | <li class="nav-item"> | ||
<!--to next page--> | <!--to next page--> | ||
| − | <a class="nav-link ico" href="https://2018.igem.org/Team:Toulouse-INSA-UPS/ | + | <a class="nav-link ico" href="https://2018.igem.org/Team:Toulouse-INSA-UPS/Demonstrate"> |
<img class="ico" src="https://static.igem.org/mediawiki/2018/3/36/T--Toulouse-INSA-UPS--All--Yohann--Right_Arrow.png" alt="Right Arrow"/> | <img class="ico" src="https://static.igem.org/mediawiki/2018/3/36/T--Toulouse-INSA-UPS--All--Yohann--Right_Arrow.png" alt="Right Arrow"/> | ||
</a> | </a> | ||
Revision as of 23:17, 12 October 2018
RESULTS
The whole Cerberus project is based on the construction and validation of a proteic platform to link very different molecules together. This platform is composed of three heads. The first head is a CBM3a domain which specifically bind cellulose. The second head is a streptavidin domain with a strong affinity to biotinylated compounds. The third head is based on the presence of an unnatural amino acid to covalently link any molecule with an alkyne moiety.
To validate the Cerberus platform, we worked head by head, starting from the CBM3a validation, then the streptavidin domain assessment and finally the demonstration of the unnatural amino acid capacity to covalently bound alkynilated molecules. Thus, our first construction has only one head and was named Sirius, after the brightest star of the northern hemisphere alpha canis majoris. The second construction presents both the CBM3a and streptavidin heads and was nicknamed Orthos, as a two-headed mythological dog. For sure, the final construction is Cerberus and its three heads.

Sirius: CBM3a
Background
Sirius is a fusion protein between CBM3a and mRFP1. This design, which consists in fusing CBM3a to a fluorescent moiety, allowed us to investigate the binding capability of the CBM3a domain to cellulose through the four aromatic amino acids of its binding domain. mRFP1 was chosen since it is both a coloured and fluorescent protein.
Key Achievements
-
Cloning of the part encoding Sirius
-
Overexpression and purification of Sirius
-
Fixation of Sirius to cellulose
Materials and Methods
The fused CBM3a and mRFP1 sequences were cloned into the pET28 expression vector in fusion with a histidine tag (Figure 1).

The resulting construct was transformed into E. coli strain and expression of the recombinant protein was induced using IPTG. The His-tagged protein was then purified on IMAC resin charged with cobalt and used in cellulose pull down assays. For the experimental details, see (link).
Results and Discussion
Production and purification of Sirius
Interestingly, E. coli turned a deep pink shade after induction, indicating a strong production of Sirius (Figure 2).


As shown in Figure 3, we successfully purified the Sirius protein. Indeed, induction with IPTG produced a large amount of a protein at the expected size for Sirius (52 kDa, lane CFE). This protein was then found predominant in elution samples (E1/40 and E1/100). We estimated the degree of purity of full-length Sirius at about 72%. In addition to the full-length protein, we observed several extra bands that likely correspond to proteolysis products.
Validation
Once produced in
When mRFP1 was added alone to cellulose, its associated colour mostly remained in the supernatant (Figure 4). When Sirius was associated to cellulose, the pinkish colour was clearly associated to the cellulose pellet. Quantification of the cellulose-associated fluorescence backed up these observations (Figure 5): control experiments showed that only background levels of fluorescence are retained in the cellulose pellet incubated with mRFP1 alone. These experiments have been performed in triplicate and statistical analyses indicate that the high level of fluorescence obtained with Sirius is definitively significant. These results clearly show that the CBM3a domain of Sirius interacts with cellulose, and thus mediates binding of mRFP1 protein to cellulose.


Orthos: Streptavidin and biotinylated compounds
Background
Next step was to assess the binding capacity of the streptavidin linker. Orthos, named after the guardian of Geryon's cattle, is a fusion protein between CBM3a and a monomeric streptavidin head. The strong affinity of streptavidin for biotin (dissociation constant of 10-13M) will allow to tightly bind biotinylated organic molecules to Orthos. We used two types of biotinylated compounds to monitor the ability of our platform to functionalize cellulose: fluorophores (mTagBFP and fluorescein) and antimicrobial peptide (scygonadin).
Key Achievements
-
Cloning of the part encoding Orthos
-
Production of Orthos carrying monomeric streptavidin
-
Production of biotinylated fluorophores in vitro and in vivo
-
Functionalization of cellulose with biotinylated compounds
Materials and Methods
The fusion between monomeric streptavidin and CBM3a was cloned into the pET28 expression vector. The resulting construct was transformed into E. coli strain BL21 and expression of the recombinant protein was induced using IPTG. For Orthos, the histidine tag present on pET28 will not be fused to the CBM3a domain since an amber Stop codon is present between them (Figure 1). The protein was therefore not purified on the IMAC column but purified on Regenerated Amorphous Cellulose (RAC) and used in cellulose pull down assays. For the experimental details, see our experiments page.

Results and Discussion
Purification of Orthos

Figure 2 presents the results of the purification of Orthos on RAC. The supernatant (S) contained all proteins produced in E. coli, and washes W1 and W2 allowed to eliminate most of non-specific interactions with cellulose. The elution step, using ethylene glycol, released the Orthos protein from RAC. The band at about 40 kDa corresponds to Orthos (expected size: 39 kDa) and the band at 15 kDa likely corresponds to a proteolysis fragment of Orthos containing only the CBM3a module.
We estimated that the purification levels of the monomeric Orthos was about 45%, which is sufficient for our validation assays.
Validation of monomeric Orthos
Validation using in vivo biotinylation
In a first set of experiments, we assessed the ability of Orthos to functionalize cellulose with a compound biotinylated in vivo. For that purpose, we took advantage of a novel system for in vivo protein biotinylation using the biotin ligase BirA. We thus co-expressed BirA and a version of the Blue Fluorescent Protein (BFP) fused to an Avitag in E. coli. The Avitag sequence is recognized by BirA for biotin ligation. We added biotin in the culture medium during IPTG induction, in order to produce and purify biotinylated BFP.
We then incubated 3.2 μM of Orthos with 32 μM of in vivo biotinylated BFP (Figure 3). For the control experiment BFP (without Orthos), the same quantity of BFP was added to have a relevant comparison. These samples were then incubated with cellulose. After three washes with Tris-HCl, fluorescence was measured in the cellulose pellets. As shown in Figure 3, fluorescence is twice more intense in Orthos sample than in the control sample containing BFP alone. These experiments have been performed in quadruplicate and a Mann Whitney statistical test indicated that the increased fluorescence signal obtained with Orthos is significant.

These results demonstrate that Orthos binds efficiently to an in vivo biotinylated compound. In addition, they show that Orthos binds to cellulose and thus provides a proof of principle that Orthos design allows functionalization of cellulose with a fluorophore through its streptavidin linker.
Validation using in vitro chemical biotinylation
In a second set of experiments, we tested the ability of Orthos to functionalize cellulose with in vitro biotinylated compounds. Since we had both an azide-functionalized fluorescein (FITC: fluorescein isothiocyanate) and biotin-DBCO (an alkyne moiety), we imagined to combine both to produce biotinylated FITC. Besides, it was a nice way to challenge this click chemistry technology. We used the newly emerging technique Cu(I)-free strain-promoted alkyne-azide click chemistry (SPAAC) allowing to couple in vitro a molecule containing a dibenzocyclooctyne (DBCO) moiety to another molecule bearing an azide function. We used this technique to ligate in vitro biotin-DBCO to an azide-functionalized fluorescein (FITC), thus leading to the expected biotinylated FITC.

Then, 58.0 μM of biotinylated FITC was incubated with 5.8 µM of Orthos protein. The same amount of FITC alone (58.0 μM) was used for the control experiment. These samples were incubated with cellulose and after five washes with Tris-HCl, fluorescence levels were measured in the cellulose pellet fractions (Figure 4). In the presence of Orthos, fluorescence in the cellulose pellet was about twice higher than in control experiments corresponding to the cellulose pellet incubated with FITC alone (without Orthos) or with the reaction buffer only. These experiments were performed in quadruplicate and a Mann Whitney statistical test indicated that the increased fluorescence signal obtained with Orthos is very significant. This result clearly shows that purified Orthos retains the ability to mediate the interaction between cellulose and a compound biotinylated in vitro. In addition, this result provides another strong evidence that Orthos allows to functionalize cellulose using a fluorescent molecule through its streptavidin linker. It also shows that we succeeded in creating biotinylated FITC by click chemistry.
Validation using scygonadin
In order to confirm that the streptavidin moiety of Orthos is able to interact with different kind of biotinylated compounds, we also tried to bind it to an antimicrobial peptide, the scygonadin. To biotinylate this compound, we used the in vivo protein biotinylation system as the one used for BFP molecule. We co-expressed BirA with a version of scygonadin fused to the Avitag in Pichia pastoris.
We then incubated Orthos with in vivo biotinylated scygonadin, and few hours later, we added cellulose (under form of a cellulose spot). After two washes with Tris-HCl, cellulose spot with Orthos-scygonadin were deposed on Petri dish pre-inoculated with a E. coli layer. We observed after incubation overnight small halos of inhibition for some samples (Figure 5).

Perspectives
We noticed that the inhibition halo was more important for the Orthos-scygonadin sample than the scygonadin alone control. Unfortunately, we repeated this test, but we did not obtain the same result. Therefore, we need to improve our experiment to prove the coupling between Orthos and biotinylated scygonadin. These results are nevertheless encouraging in the perspective of functionalizing cellulose using Orthos bound to antibiotic protein.
Perspectives
Our experiments using different kinds of biotinylated compounds provide convincing proofs of concept that Orthos can be conjugated, via its streptavidin head, to biotinylated organic molecules. The resulting molecules interact with cellulose and are therefore potent cellulose functionalizing compounds.
We can now envision to exploit the streptavidin linker of Orthos to ligate various compounds that can be biotinylated chemically (in vitro) or in vivo, via BirA co-expression.
In addition, we incorporated a TEV protease cleavage between the N-terminus linker and the streptavidin. This will allow to produce Orthos-bound biotinylated compounds that can be released by TEV cleavage if required, as in the case of odorant molecules for examples for a commercial use.
Cerberus: AzF
Background
Cerberus, named after the guardian of the gates of the Underworld, is our three-headed protein made of the cellulose-binding molecular platform (CBM3a) fused at the N-terminus to the biotinylated molecule-binding head (monomeric streptavidin) and at the C-terminus to the unnatural amino acid azido-L-phenylalanine (AzF). The incorporation of AzF is achieved through the recognition of the amber stop codon at the C-terminus of CBM3a by an orthogonal AzF-charged tRNA.

Coupling of molecules to the AzF head can be performed by click chemistry, either through SPAAC or through Cu(I)-catalyzed azide-alkyne click chemistry (CuAAC). To prove the versatility of the system, different compounds such as fluorescent proteins or paramagnetic beads have been clicked to Cerberus and thus associated to cellulose.
Key Achievements
-
Cloning of the part encoding Cerberus
-
Production of Cerberus
-
Functionalization of cellulose
Materials and Methods
The fusion between the streptavidin linker and the CBM3a domain sequences followed by the amber stop codon was cloned into the pET28 expression vector in fusion with the His-tag. The resulting construct was co-transformed into E. coli strain with the pEVOL expression vector. The latter allows to express the amino acyl tRNA synthetase aaRS. In presence of AzF, this enzyme synthesizes the orthogonal AzF-tRNA for in vivo AzF incorporation in place of amber Stop codon. The Cerberus platform was produced by adding IPTG (Cerberus induction), L-arabinose (amino acyl tRNA synthetase production), and AzF to the medium. The His-tagged protein was then purified on IMAC resin charged with cobalt and used in cellulose pull down assays. For the experimental details, see experiments page.
Results and Discussion
Production and purification of Cerberus
Cerberus production requires a deferred induction. We first induced the synthesis of aarS/tRNA pair with L-arabinose, and added AzF at the same time. One hour later, we induced the production of Cerberus with IPTG.

Cerberus production and purification were analysed on SDS gel (Figure 2). Induction in the presence of AzF (Figure 2, lane I+AzF) led to the expression of two major bands at 37 and 39 kDa approximately compared to control conditions, namely the non-induced (lane NI) and induced without AzF (lane I-AzF). We hypothesized that the upper band would correspond to Cerberus (expected molecular weight of Cerberus with monomeric streptavidin: 41 kDa) and the lower bands to the Orthos proteins (Cerberus without the AzF and the His-tag). Surprisingly, both bands were present in the elution fractions which is incoherent since Orthos lacks an His-tag and was supposed to be washed out during purification steps. To confirm our hypothesis, we analysed the same fractions by western blot using antibodies detecting the His-tag (Figure 3).

Anti-His-tag antibodies revealed a band at about 45 kDa in the sample corresponding to IPTG induction in the presence of AzF (Figure 3, lane I+AzF). This band is not present in control samples (Figure 3, lane NI and I-AzF), indicating that it corresponds to the Cerberus protein (theoretical size: 41 kDa). In addition to the full length protein, we observed several extra bands which very likely correspond to proteolysis products since they are detected with the anti-His-tag antibodies. Moreover, the band at 45 kDa is clearly detected in elution samples (Figure 3, lanes E). The band at 37 kDa observed on the SDS PAGE (Figure 2) is not observed on the Western blot. These results show that the experimental setup to produce Cerberus is also likely leading to the production of Orthos when the amber stop codon is not recognized by the AzF-charged orthogonal tRNA. Although Orthos does not contain a His-tag at its C-terminus, the protein seems to be efficiently co-purified with Cerberus. The basis of this observation is unclear but it may suggest that Orthos and Cerberus interact with each other, via their CBM3 or streptavidin moieties.
We estimated that the purification level of Cerberus with monomeric streptavidin in the elution samples was about 62%. These data show that Cerberus was efficiently purified and can be used for pull down assays.
Validation
Validation using FITC (Fluorescein isothiocyanate) molecules
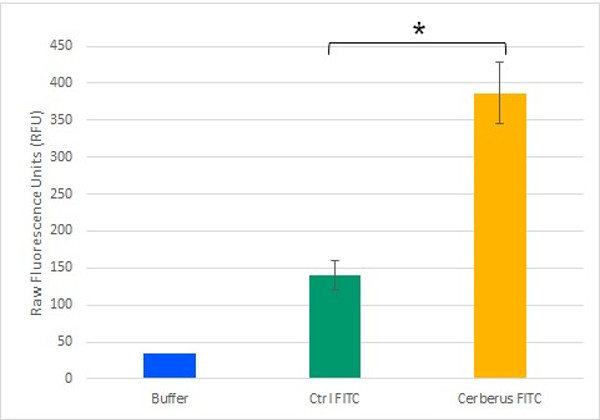
To test the potential of Cerberus in functionalizing cellulose, we monitored its ability to mediate an interaction between cellulose and a fluorescent compound. To generate a fluorescently labelled Cerberus protein, we performed a SPAAC reaction on 3.2 µM Cerberus protein using 31.9 µM µg of FITC-DBCO. In control experiment, 31.9 µM of FITC alone was used. These samples were then incubated with cellulose and after five washes with Tris-HCl, fluorescence levels were measured in the cellulose pellet fractions (Figure 9). We observed that the fluorescence levels in the cellulose pellet incubated with Cerberus protein clicked to FITC were about 3 times higher than in the control experiments corresponding to the cellulose pellet incubated with FITC alone. These results show that Cerberus can efficiently be conjugated to fluorescent molecules bearing a DBCO group by click chemistry and that the resulting fluorescent molecules strongly interact with cellulose. Therefore, Cerberus is both a convenient and potent platform to functionalize cellulose.
Validation using paramagnetic beads
We further characterize the functionality of Cerberus in a second set of experiments using paramagnetic beads. To bind paramagnetic beads to Cerberus, we performed a click reaction using 3.2 µM of Cerberus protein and 32 µM DBCO-conjugated paramagnetic beads. As a control experiment, 32 µM of paramagnetic beads were used. These samples were then incubated with cellulose, and after five washes with Tris-HCl, we measured the magnetisation of cellulose using a magnet.
As observed on the video (Figure 5), the cellulose incubated with the Cerberus protein conjugated to paramagnetic beads is quickly and totally attracted by the magnet. In contrast, in the control experiment, no clear movement towards the magnet was observed.
These results show that Cerberus has the ability to interact simultaneously with cellulose and molecules with a DBCO group, confirming that the Cerberus platform allows to functionalize cellulose through its linker containing unnatural amino acid.
Compounds functionalization
To go further in our project, we decided to functionalise two inorganic molecules, graphene and carbon nanotubes (CNT), using a reaction of diazotization. This experiment was performed with the help of the ENSIACET laboratory in Toulouse. To check functionalization, we analysed samples by Thermogravimetric Analysis (TGA). The concept of TGA consists in measuring the mass change of a sample depending on temperature and time, in a controlled atmosphere. We chose to perform the experiment under azote, an inert gas that avoids mass loss by oxidation, and we studied the mass change from 25 to 1,000 °C (15 °C/min).


Figures 6 and 7 present the results of mass loss for non-functionalized Carbon Nanotube (CNT) and functionalized CNT samples, respectively. For functionalized CNT sample (Figure 7), we observed a mass loss of 27.4% around 100 °C corresponding to water loss. A second step of mass loss is observed around 550 °C, with a diminution of 19.8%. For the non-functionalized sample (Figure 6), we observed two mass losses at 150 and 750 °C corresponding to 1.5% and 3% of mass loss respectively. The significative difference between these two samples allowed us to conclude that the CNT functionalization has been successful.


Figures 8 and 9 present the results of mass loss for non-functionalized graphene and functionalized graphene samples respectively. For functionalized graphene sample (Figure 9), we observed a mass loss of 31.0% around 100 °C corresponding to water loss. A second step of mass loss is observed around 500 °C, with a diminution of 19.0%. For the non-functionalized sample (Figure 8), we did not observe a significative mass loss. So we can conclude that the graphene functionalization has been successful.
Our initial plan was to functionalize cellulose with CNT and graphene in order to change cellulose fibre rigidity and create conductive cellulose, respectively. Unfortunately, we did not have enough time during the course of the iGEM competition to complete these tests. Given the success of the other functionalization, we have reasonable hopes that these experiments will be successful too.
Perspectives
Our experiments provide robust proofs of concept that Cerberus can be conjugated, via its unnatural amino acid AzF, to organic or inorganic molecules bearing a DBCO group by click chemistry. The resulting molecules strongly interact with cellulose and are therefore potent cellulose functionalizing compounds.
We can now consider fixation of various compounds that can be chemically functionalized. We can also imagine double fixations, for example a fluorophore on the streptavidin head and paramagnetic beads on the AzF linker. This double fixation allows to envision endless possibilities to functionalize cellulose (see Demonstrate).
Bacterial Cellulose production and functionalization
Background
Our ultimate dream was to be able to produce functionalized cellulose in vivo. Some microorganisms naturally produce cellulose. One of these, Gluconacetobacter hansenii, can produce cellulose as a biofilm to protect itself. We took advantage of the work of iGEM Imperial team 2014 who has set up culture conditions to optimize cellulose production. Our challenge was to assess if our technology is compatible with bacterial cellulose as a first step before producing functionalized cellulose in vivo.
Key Achievements
-
Production of about 100g of dried bacterial cellulose
-
Functionalization of bacterial cellulose with Sirius
Materials and Methods
G. hansenii was grown in Hestrin-Shramm (HS) medium at 30 °C. After one week of culture, bacterial cellulose was extracted and purified. For the experimental details, see our experiments page.
For the functionalization, bacterial cellulose was incubated with purified Sirius (CBM3a-mRFP1) or with purified mRFP1 alone as a control. Cellulose was then washed about 10 times with Tris-HCl buffer to remove non-attached proteins.
Results and Discussion


Figure 1 presents the results of the functionalization of our bacterial cellulose with the Sirius fluorescent protein. Only the cellulose sample incubated with Sirius kept the color after several washes with resuspension buffer. Likewise fluorescence remained associated to cellulose only for Sirius (Figure 2). These results show that CBM3a can interact with the bacterial cellulose and allows fixation of the mRFP1 protein to cellulose.
Perspectives
Now that we proved the capacity of CBM3a to interact with bacterial cellulose, we are confident that Orthos and Cerberus proteins should also interact with bacterial cellulose. Now the next step will be to produce in vivo functionalized bacterial cellulose. For that, we had planified to produce the Cerberus platform in the yeast Pichia pastoris because of its secretion capacity. Indeed, our idea was to co-culture both strain in the same reactor in order to produce functionalized cellulose in vivo. This work on P. pastoris was performed in the same time than our effort in E. coli but has not been as successful. We managed to produce the Orthos protein in the yeast but we never succeeded in producing Sirius and we never got the plasmid mendatory to produce the tRNA synthetase necessary for AzF incorporation in P. pastoris. Beside, the production of Orthos in the yeast was very low and not sufficient to conduct our assays. So production of functionalized in vivo will require to improve the P. pastoris part of our project.
No dogs were harmed over the course of this iGEM project.
The whole Toulouse INSA-UPS team wants to thank our sponsors, especially:









And many more. For futher information about our sponsors, please consult our Sponsors page.
The content provided on this website is the fruit of the work of the Toulouse INSA-UPS iGEM Team. As a deliverable for the iGEM Competition, it falls under the Creative Commons Attribution 4.0. Thus, all content on this wiki is available under the Creative Commons Attribution 4.0 license (or any later version). For futher information, please consult the official website of Creative Commons.
This website was designed with Bootstrap (4.1.3). Bootstrap is a front-end library of component for html, css and javascript. It relies on both Popper and jQuery. For further information, please consult the official website of Bootstrap.



