| (5 intermediate revisions by 2 users not shown) | |||
| Line 58: | Line 58: | ||
<li><a href="https://2018.igem.org/Team:UST_Beijing/Attributions">Attribution</a ></li> | <li><a href="https://2018.igem.org/Team:UST_Beijing/Attributions">Attribution</a ></li> | ||
<li><a href="https://2018.igem.org/Team:UST_Beijing/Collaborations">Collaboration</a ></li> | <li><a href="https://2018.igem.org/Team:UST_Beijing/Collaborations">Collaboration</a ></li> | ||
| − | <li><a href="https:// | + | <li><a href="https://static.igem.org/mediawiki/2018/5/5a/T--UST_Beijing--Demonstrate.pdf">Demonstrate</a ></li> |
<li><a href="https://2018.igem.org/Team:UST_Beijing/InterLab">InterLab</a ></li> | <li><a href="https://2018.igem.org/Team:UST_Beijing/InterLab">InterLab</a ></li> | ||
<li><a href="https://2018.igem.org/Team:UST_Beijing/Human_Practices">HumanPractice</a ></li> | <li><a href="https://2018.igem.org/Team:UST_Beijing/Human_Practices">HumanPractice</a ></li> | ||
| Line 117: | Line 117: | ||
<div class="pad45"></div> | <div class="pad45"></div> | ||
</div> | </div> | ||
| − | + | ||
| + | </div> | ||
| + | <div class="row"> | ||
| + | <div class="span6"> | ||
<h2><span>Nuclear receptor LXR as therapeutic target against atherosclerosis</span></h2> | <h2><span>Nuclear receptor LXR as therapeutic target against atherosclerosis</span></h2> | ||
<h3>Live X receptor(LXR) functions as a master regulation switch, which can accelerate the transport of cholesterol and prevent the formation of foam cells.</h3> | <h3>Live X receptor(LXR) functions as a master regulation switch, which can accelerate the transport of cholesterol and prevent the formation of foam cells.</h3> | ||
| Line 123: | Line 126: | ||
<div class="hover_colour"> <a href="https://static.igem.org/mediawiki/2018/4/4d/T--UST_Beijing--bg9.jpg" data-rel="prettyPhoto[portfolio1]"> <img src="https://static.igem.org/mediawiki/2018/f/fc/T--UST_Beijing--bg7.jpg" alt="" /></a> </div> | <div class="hover_colour"> <a href="https://static.igem.org/mediawiki/2018/4/4d/T--UST_Beijing--bg9.jpg" data-rel="prettyPhoto[portfolio1]"> <img src="https://static.igem.org/mediawiki/2018/f/fc/T--UST_Beijing--bg7.jpg" alt="" /></a> </div> | ||
</div> | </div> | ||
| − | + | ||
<div class="span6 pad10"> | <div class="span6 pad10"> | ||
| − | <h2><span> | + | <h2><span> Actions of LXR </span></h2> |
| − | <h3>LXRs | + | <h3>LXRs have a typical nuclear receptor structure. Upon bound by agonists (such as ginseno-sterols), LXRs would recruit co-activators (shown in the animation, only a fragment of coactivator peptide is displayed)and activate the transcription of a target gene.</h3> |
<div class="vendor"> | <div class="vendor"> | ||
<iframe src="https://static.igem.org/mediawiki/2018/b/bc/T--UST_Beijing--bgbg.mp4" height="500"></iframe> | <iframe src="https://static.igem.org/mediawiki/2018/b/bc/T--UST_Beijing--bgbg.mp4" height="500"></iframe> | ||
| Line 136: | Line 139: | ||
<div class="row"> | <div class="row"> | ||
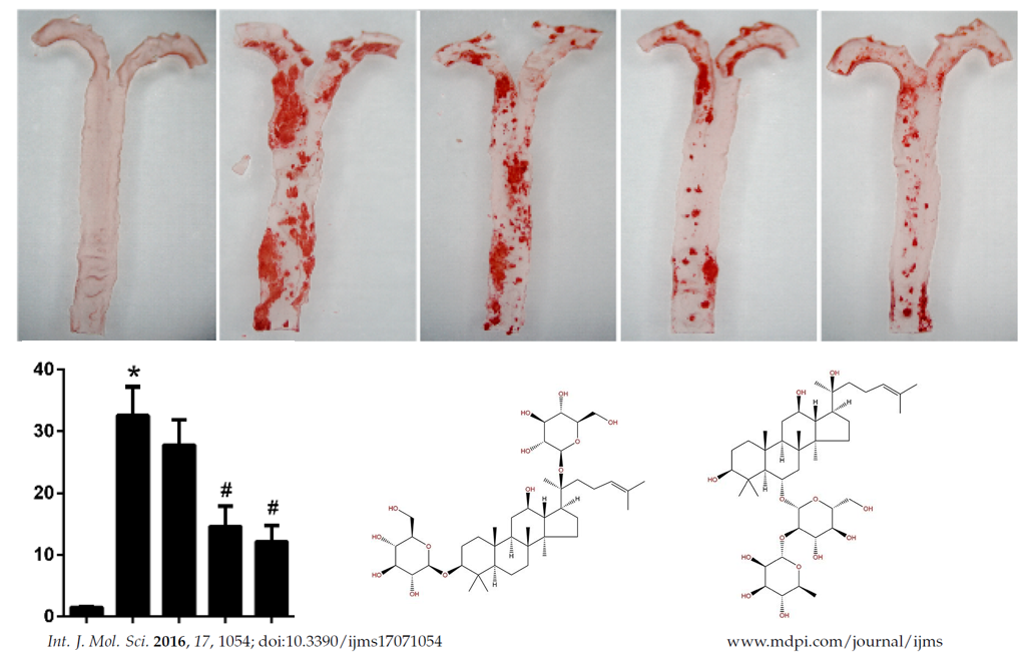
<h3>Ginseng triterpenes' structure is similar to that of cholesterol, therefore it has the potential of regulating LXRs. | <h3>Ginseng triterpenes' structure is similar to that of cholesterol, therefore it has the potential of regulating LXRs. | ||
| − | LXRs is a typical nuclear receptor. In the presence of co-regulators, bound by agonists (such as ginsenosides), LXRs could be demobilized to | + | LXRs is a typical nuclear receptor. In the presence of co-regulators, bound by agonists (such as ginsenosides), LXRs could be demobilized to specific site on a target gene promoter region to activate transcription of the target gene. Ginsenosides or like could therefore regulate the metabolism of cholesterol. </h3> |
</div> | </div> | ||
Latest revision as of 15:28, 16 October 2018
Cholesterol
an essential component of membrane structure, but gradually accumulates in arteries of human body, which becomes the top one killer mechanism of human life: atherosclerosis
An alarming health threat: Atherosclerosis
Shown on the right are the cholesterol structure and pie charts of epidemic statistics of atherosclerosis-related mortality in China (black area: atherosclerosis-related death).