| Line 78: | Line 78: | ||
<p class="about-para">Guided by the successful chemotaxis results (link), proving that 50µM naringenin attracts the nitrogen fixing bacteria <i>A. brasilense</i> and <i>H. seropedicae</i>, we aimed to engineer a naturally colonising endophyte <i>Pseudomonas sp.</i> (CT 364) to produce naringenin. For proof of concept the production of naringenin would first need to be demonstrated in <i>E. coli</i> before being tested in <i>Pseudomonas sp.</i>, our final chassis organism.</p> | <p class="about-para">Guided by the successful chemotaxis results (link), proving that 50µM naringenin attracts the nitrogen fixing bacteria <i>A. brasilense</i> and <i>H. seropedicae</i>, we aimed to engineer a naturally colonising endophyte <i>Pseudomonas sp.</i> (CT 364) to produce naringenin. For proof of concept the production of naringenin would first need to be demonstrated in <i>E. coli</i> before being tested in <i>Pseudomonas sp.</i>, our final chassis organism.</p> | ||
<p class="about-para">Naringenin biosynthesis is achieved through the expression of an operon containing four genes encoding the enzymes that constitute the naringenin biosynthetic pathway (Figure 1). This operon was previously assembled and submitted to the iGEM registry by TU Darmstadt 2014 iGEM team (BBa_K1497016 (link)) and is a composite of the following four genes, each with the strong RBS (BBa_B0034): | <p class="about-para">Naringenin biosynthesis is achieved through the expression of an operon containing four genes encoding the enzymes that constitute the naringenin biosynthetic pathway (Figure 1). This operon was previously assembled and submitted to the iGEM registry by TU Darmstadt 2014 iGEM team (BBa_K1497016 (link)) and is a composite of the following four genes, each with the strong RBS (BBa_B0034): | ||
| − | + | <ul>4-Coumaryl ligase - 4CL (BBa_K1033001) </ul> | |
| − | + | <ul>Tyrosine ammonia lyase - TAL (BBa_K1033000) </ul> | |
| − | + | <ul>Chalcone isomerase - CHI (BBa_K1497000)</ul> | |
| − | + | <ul>Chalcone synthase - CHS (BBa_K1497001)</ul> | |
| − | We decided to optimise the sequence for the enzyme tyrosine ammonia lyase by reducing the G/C content so that it could be synthesised by IDT. The entire operon was synthesised in four separate parts referred to as gBlocks that were subsequently used for Gibson Assembly, as this was how TU Darmstadt assembled their operon successfully. Moreover this assembly does not leave restriction site scars due to the overlapping fragments. Instead of using Pseudomonas sp. it was deemed logical to use E. | + | We decided to optimise the sequence for the enzyme tyrosine ammonia lyase by reducing the G/C content so that it could be synthesised by IDT. The entire operon was synthesised in four separate parts referred to as gBlocks that were subsequently used for Gibson Assembly, as this was how TU Darmstadt assembled their operon successfully. Moreover this assembly does not leave restriction site scars due to the overlapping fragments. Instead of using <i>Pseudomonas sp.</i> it was deemed logical to use <i>E. coli</i> DH5α during the cloning experiments as we had a greater understanding of its transformation. Using this as a proof of concept would allow us to carry out a preliminary screening for naringenin production before attempting to transform our endophyte.</p> |
| − | <p class="about-para">Alongside this, in an attempt to optimise naringenin production, a new design of the naringenin operon was made in Benchling. This was based on the pathway modelling results (link) and was constructed to show how BG28 and BG51 dual E. coli-Pseudomonas promoters with a 10-fold difference in strength could increase naringenin production.</p> | + | <p class="about-para">Alongside this, in an attempt to optimise naringenin production, a new design of the naringenin operon was made in Benchling. This was based on the pathway modelling results (link) and was constructed to show how BG28 and BG51 dual <i>E. coli-Pseudomonas</i> promoters with a 10-fold difference in strength could increase naringenin production.</p> |
</div> | </div> | ||
Revision as of 09:34, 17 October 2018
Alternative Roots
Naringenin Operon Assembly Results
Results
Introduction
Guided by the successful chemotaxis results (link), proving that 50µM naringenin attracts the nitrogen fixing bacteria A. brasilense and H. seropedicae, we aimed to engineer a naturally colonising endophyte Pseudomonas sp. (CT 364) to produce naringenin. For proof of concept the production of naringenin would first need to be demonstrated in E. coli before being tested in Pseudomonas sp., our final chassis organism.
Naringenin biosynthesis is achieved through the expression of an operon containing four genes encoding the enzymes that constitute the naringenin biosynthetic pathway (Figure 1). This operon was previously assembled and submitted to the iGEM registry by TU Darmstadt 2014 iGEM team (BBa_K1497016 (link)) and is a composite of the following four genes, each with the strong RBS (BBa_B0034):
- 4-Coumaryl ligase - 4CL (BBa_K1033001)
- Tyrosine ammonia lyase - TAL (BBa_K1033000)
- Chalcone isomerase - CHI (BBa_K1497000)
- Chalcone synthase - CHS (BBa_K1497001)
Alongside this, in an attempt to optimise naringenin production, a new design of the naringenin operon was made in Benchling. This was based on the pathway modelling results (link) and was constructed to show how BG28 and BG51 dual E. coli-Pseudomonas promoters with a 10-fold difference in strength could increase naringenin production.

Figure 1: The naringenin synthesis pathway from L-tyrosine.
Results
Experimental Work
Plasmid Design
The naringenin operon contains the genes for four enzymes: 4 – Coumaryl ligase, Tyrosine ammonia lyase, Chalcone isomerase and Chalcone synthase. Each of these genes will contain a strong ribosome binding site and has an Escherichia coli his operon terminator. This construct will be under the control of a J23100 constitutive promoter to observe its expression in E.coli as a proof of concept. Once biosynthesis under the control of J23100 is achieved, the construct future experiments would test this under a T7 promoter in E. coli. Parts for biosynthesis in the final chassis organism, root-colonising Pseudomonas sp., will be constructed in the plasmid backbone pSB1C3.
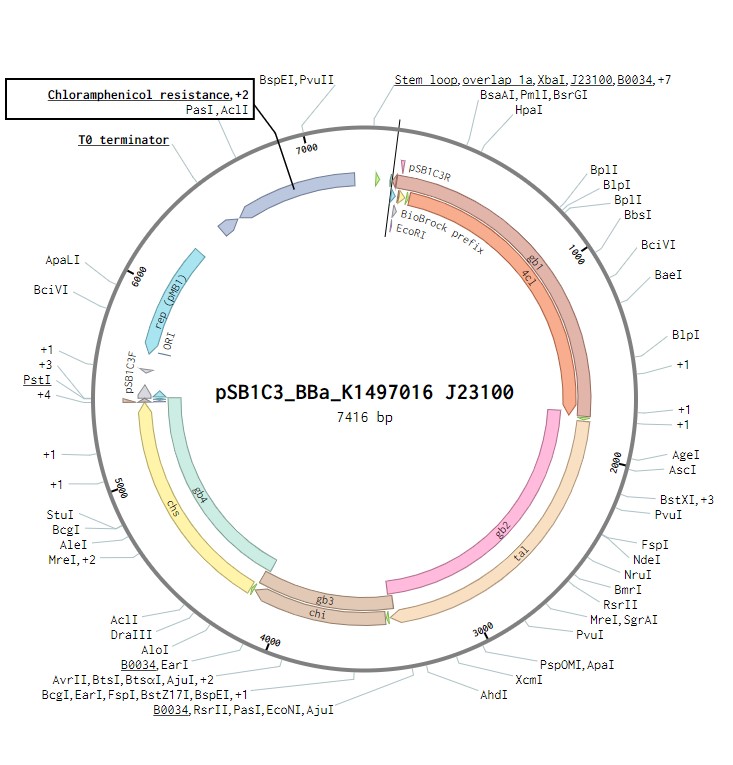
The naringenin biosynthetic operon under control of a J23100 promoter created in Benchling.

The naringenin biosynthetic operon under control of a T7 promoter created in Benchling.

The naringenin biosynthetic operon construct under control of a J23100 promoter created in SBOL.

The naringenin biosynthetic operon contruct under control of a T7 promoter created in SBOL.
Naringenin pathway modelling influenced design
Results of the naringenin pathway modelling showed that weaker expression of the first two genes and 10 fold stronger expression of the last two genes would reduce the build-up of malonyl CoA and optimise naringenin synthesis. Therefore we found two synthetic promoters: BG28 and BG51 and an additional E. coli his operon terminator to be placed after the first two genes. These will allow enhanced naringenin production in future experiments, as BG28 is a weak promoter for the first two genes and BG51 is a strong promoter for the last two. More information about the pathway can be found here.
The naringenin biosynthetic operon under control of synthetic promoters BG28 and BG51 created in Benchling.

A close up of the synthetic promoters BG28 and BG51 placement in the operon, created in Benchling.

The naringenin biosynthetic operon contruct under control of a BG28 and BG51 promoters and an additional his operon terminator created in SBOL.
Table 1: Primers designed in Benchling for amplification and detection of the 4 gblocks and the pSB1C3 backbone.
| Primer name | Sequence | Tm | Ta Q5 | Amplified product(bp) | Shown to work | Description |
|---|---|---|---|---|---|---|
| pSB1C3F | tactagtagcggccgctgc | 70 | 71 | 2070 | 6/8/18 | To amplify pSB1C3 backbone |
| pSB1C3R | ctctagaagcggccgcga | 70 | 71 | 2070 | 6/8/18 | To amplify pSB1C3 backbone |
| 4CLF | ccaaatcgccgccaattttc | 59 | 56 | 1686 | 6/9/18 | To amplify 4CL part |
| 4CLR | cgtcgtcgttttgaagtggt | 59.07 | 56 | 1686 | 28/9/18 | To amplify 4CL part |
| TALF | gaatgtccgaacgctacagg | 58.72 | 55 | 1649 | 28/9/18 | To amplify TAL part |
| TALR | tcggaattgagcaggtcgat | 59.18 | 56 | 1649 | 28/9/18 | To amplify TAL part |
| CHIF | ctgggcatagaggtctggag | 58.95 | 56 | 726 | 28/9/18 | To amplify CHI part |
| CHIR | caccttctccgagtactgct | 58.82 | 56 | 726 | 28/9/18 | To amplify CHI part |
| CHSF | aagacgtgcctgggttgata | 59.02 | 56 | 1197 | 28/9/18 | To amplify CHS part |
| CHSR | gcttctcctccttcaaccct | 59.01 | 56 | 1197 | 6/9/18 | To amplify CHS part |
| gb1F | ctggaattcgcggccgct | 72 | 54 | 1686 | 20/9/18 | To amplify 4CL part |
| gb1R | ttacaatccatttgctag | 53 | 54 | 1686 | 20/9/18 | To amplify 4CL part |
| gb2F | ggcaaaactagcaaatgg | 59 | 59 | 1649 | 20/9/18 | To amplify TAL part |
| gb2R | ttatcagacgggagattg | 58 | 59 | 1649 | 20/9/18 | To amplify TAL part |
| gb3F | cttgcagcaatctcccgt | 65 | 59 | 726 | 20/9/18 | To amplify CHI part |
| gb3R | ctagactccaatcactgg | 58 | 59 | 726 | 20/9/18 | To amplify CHI part |
| gb4F | tactattccagtgattgg | 54 | 55 | 1197 | 20/9/18 | To amplify CHS part |
| gb4R | cggactgcagcggccgct | 78 | 55 | 1197 | 20/9/18 | To amplify CHS part |
Backbone amplification
The backbone was amplified, purified and quantified to prepare for Gibson assembly, its stock concentration was found to be 26.2 µg/ ml. This would allow the overlapping ends of the gblocks to ligate to the plasmid backbone. The amplification was done by PCR, purified using QIAquick PCR Purification Kit (250) and quantified by use of a Qubit fluorometer.
Protocol Details found hereGibson Assemblies
Positive control of the Gibson Assembly was conducted using the NEBuilder HiFi Assembly mix and the positive control reagent containing 2 overlapping dsDNA fragments for control assembly and the pUC19 control DNA plasmid. This was conducted to check the assembly mix was working, the protocols for transformation were correct and that the competent cells made on the 10/8/18 had been induced to be competent. The results of this positive control Gibson assembly showed that the DH5 alpha cells had successfully been made competent as they were able to take up the three part assembly. It also showed that the reagents and protocols to be used in future Gibson assemblies of the naringenin operon worked. Using ampicillin LB plates it could be concluded that the colonies growing have taken up the three part assembly, as the plasmid backbone contained ampicillin resistance.
Figure 3: Positive and negative control ampicillin plates for the positive control fragments for Gibson Assembly.
Results
Conclusions
Two colonies from Gibson transformations resulted in a 7kb band, corresponding to the size of the operon and plasmid backbone. However sequencing was inconclusive therefore this operon could not be classified as a working part. Future experimentation should attempt to assemble the gblocks one by one into the plasmid backbone and gain sequencing results that show full alignment. Following this the 7kb plasmid should be transformed into BL21 expression cells to produce naringenin, to be extracted using ethyl acetate and measured through HPLC. HPLC of stock naringenin should be conducted for comparison. Observing the expression of the operon using T7 promoters instead of the J23100 constitutive promoter, to see if naringenin production is enhanced. This could be implemented using T7 primers and Q5 site directed mutagenesis in E. coli.




