
To summarise and validate the successful construction of the parts comprising the Chlorophyll Biosynthesis Plasmid, all composite parts underwent single (EcoRI) and double (EcoRI with PstI) digests followed by agarose gel (0.5%) electrophoresis. The results from the digests showing the successful assembly of the Chlorophyll Biosynthesis Plasmid are shown in Figure 1 (below).
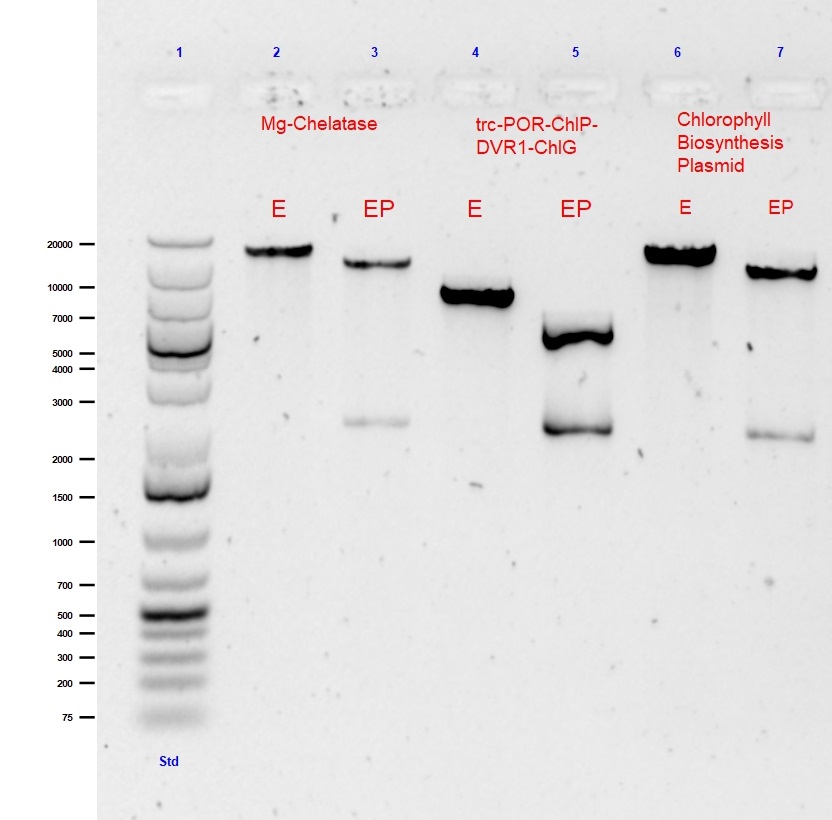
This large 9.3kB biobrick of 5 genes was created to begin the 1st step of the Chlorophyll biosynthesis pathway away from Heme biosynthesis utilising the cells naturally produced PPIX to Mg-PPIX. Standard assembly of trc-ChlI1-ChlI2-ChlD and trc-ChlH-GUN4 was performed in a CAM biorick backbone. The single and double digest of this Mg-chelatase plasmid is shown in lanes 2 and 3 of Figure 1, respectively.
The second large 4.7kB biobrick construct called “PPDG” was created as the final part of our Chlorophyll A biosynthesis. This composite part was created to convert photoxidative PChlide to Chlorophyll A. This was important as it allowed for stability of our light triggered vesicle formation system. This part was formed using Standard assembly of trc-POR-ChlP and DVR1-ChlG into a CAM backbone.The single and double digest of this trc-POR-ChlP-DVR1-ChlG plasmid is shown in lanes 4 and 5 of Figure 1, respectively.
The final construct is the assembly of the whole “CBP Biobrick”. This is 18.3kB composite part was pieced together using all 14 genes of our Chlorophyll biosynthesis pathway into the CAM backbone. This part was formed using Standard assembly of previously assembled Mg-Chelatase plasmid and lac-CTH1-ycf54-ChlM-trc-FNR-fdx-trc-POR-ChlP-DVR1-ChlG.The single and double digest of our pièce de résistance CBP plasmid is shown in lanes 6 and 7 of Figure 1, respectively.
The POR gene with the trc promoter showed a high level of inducible expression by IPTG within the KAN backbone (Figure 2). The POR protein is seen clearly at the expected size of 40 kDa. This result validates the expression of POR under the new trc promoter.

Protochlorophyllide (Pchlide) was extracted from barley (Figure 3) and used as a substrate for to assess the function of the expressed POR. The barley extracted Pchlide was added to the lysate of the IPTG induced- E.coli containing the trc-POR construct. The mixture was incubated for varying times (0 to 30 mins) then placed in a spectrophotometer. As shown in Figure 4, an absorbance at 627-630 nm shows the presence of protochlorophyllide. An absorbance peak at 665-670 nm would be expected for POR activity but this could not be seen.We believe a lack of a POR peak at 665-670nm may be due to the presence of other cell proteins in the crude cell lysate that interfere with our assay. Literature on this assay has stated that the assay requires purified POR protein (Townley et al, FEBS Letters 422: 19-22 1988). We continue to work on the purification of our expressed protein using chromatography methods.


Using a fluorescence spectroscopy assay we checked for production of Mg-PPIX using our induced Mg-Chelatase plasmid. The sample was excited with an excitation wavelength of 420nm. Purified proteins of Mg-Chelatase (provided by our advisor Professor Robert Willows) was used as a positive control.. A peak appearing at 595nm shows successful production of Mg-PPIX, as demonstrated by our positive control (Figure 5). We assayed our induced Mg-Chelatase plasmid by adding purified individual protein subunits of Mg-Chelatase to see if the expression of any individual gene was limiting. No added individual protein subunit demonstrated an enhancement on the expression of our Mg-PPIX using our Mg-Chelatase plasmid. We proceeded by adding ChlI1,ChlI2 and ChlD to our Mg-Chelatase plasmid lysate. This was performed to see if we could characterise the expression of ChlH. This yielded similar results. Our findings are also supported by the qPCR expression results provided by our collaboration with NTU iGEM team that showed a decrease in expression of genes after induction by ITPG and inability to characterise ChlH and ChlD expression(Results shown in our collaboration page). We are continuing to investigate the reasons for this unexpected decrease in expression.






