ThomasStarck (Talk | contribs) |
|||
| (103 intermediate revisions by 12 users not shown) | |||
| Line 9: | Line 9: | ||
background-color: #292929; | background-color: #292929; | ||
} | } | ||
| − | # | + | #bannerchanged{ |
| − | + | width: 100%; | |
| + | overflow: hidden; | ||
| + | margin: 0 auto; | ||
} | } | ||
@media screen and (max-width: 760px) { | @media screen and (max-width: 760px) { | ||
| − | # | + | /*#wrapper { |
| − | + | width: 100%; | |
| + | overflow: hidden; | ||
| + | } | ||
| + | #container { | ||
| + | width: 100%; | ||
| + | margin: 0 auto; | ||
| + | }*/ | ||
| + | .banner-img { | ||
| + | width: 100%; | ||
} | } | ||
} | } | ||
| − | + | ||
| − | + | ||
| − | + | ||
</style> | </style> | ||
| − | <div id="banner"> | + | <div id="bannerchanged"> |
| − | <h1> | + | <img class="banner-img" src="https://static.igem.org/mediawiki/2018/7/76/T--Pasteur_Paris--Banner_IHP.jpg"> |
| + | </div> | ||
| + | <h1></h1> | ||
</div> | </div> | ||
| − | |||
<div id="GeneralContent"> | <div id="GeneralContent"> | ||
| Line 48: | Line 57: | ||
<div class="block title" style="margin-top: 40px"><h3 style="text-align: left;">Developing a Project Idea</h3></div> | <div class="block title" style="margin-top: 40px"><h3 style="text-align: left;">Developing a Project Idea</h3></div> | ||
<div class="block full"> | <div class="block full"> | ||
| − | <p>After assembling our team, we spent the first two months in brainstorming sessions, researching potential topics for this year’s competition. A team member presented us the field of bionic | + | <p>After assembling our team, we spent the first two months in brainstorming sessions, researching potential topics for this year’s competition. A team member presented us the field of bionic prostheses, and we were all shocked by the number of people who were suffering from an amputation. His first idea was to use the capacity of bacteria to drive an electrical current to amplify the signal between the patient’s nerves and electrical sensors linked to a prosthesis. This way, redirection of nerves would not be necessary and the patient would be able to accomplish more natural actions. </p> |
| − | <p>When we started to look more in details the field of prosthesis and | + | <p>When we started to look more in details the field of prosthesis and implants, we realized that along with device loosening or malfunctions and foreign-material reactions, infection remains the most serious problems encountered with surgical implants. We quickly learned that biofilm formation is common to all types of implanted foreign-body infections. Indeed, the high susceptibility of implanted devices to infection is due to a locally acquired host defense deficiency. Thus, this persistence at a specific site is mainly caused by the rapid formation of a biofilm, which is resistant to host defense and antimicrobial agents as a result of reduced access and diffusion characteristics within it. We then decided to integrate this aspect inside our project: while favoring the growth of the nerve and the conduction of a signal through a bacterial interface, we could also diminish the risk of infection. At first, we wanted to find a system to directly kill the <i>Staphylococcus aureus</i>, but after talking with Pr. Jean-Marc Ghigo (Genetics of Biofilm Unit, Institut Pasteur), we realized that the biofilm configuration would give us some hard time and require extra steps to manipulate. We decided to shift our goal, and rather than killing <i>S. aureus</i>, we would limit the virulence of the bacteria and restrict its ability to form a biofilm. This could be achieved by subverting the quorum sensing of the pathogenic bacteria and blocking the signal. In this way, <i>S. aureus</i> could be handled by the host’s immune system and by the patient’s doctor with a normal dosage of antibiotics.</p> |
<p>In order to learn more about the significance of these issues in the medical field, we interviewed many professionals working either directly with patients, industries working on high tech metallic or ceramic implants, and amputees through the contact of ADEPA « Association for the Defense and Study of Amputated People ». Through all this work, we were invited for many tours, first at the European Hospital George-Pompidou where we had the chance to observe Dr. Benjamin Bouyer M.D., a lumbar rachis surgeon, during surgery to see the procedures put in place to diminish the risk of infection during the integration of an implant inside the body.</p> | <p>In order to learn more about the significance of these issues in the medical field, we interviewed many professionals working either directly with patients, industries working on high tech metallic or ceramic implants, and amputees through the contact of ADEPA « Association for the Defense and Study of Amputated People ». Through all this work, we were invited for many tours, first at the European Hospital George-Pompidou where we had the chance to observe Dr. Benjamin Bouyer M.D., a lumbar rachis surgeon, during surgery to see the procedures put in place to diminish the risk of infection during the integration of an implant inside the body.</p> | ||
</div> | </div> | ||
| − | <div class="block title"><h3 style="text-align: left;"> | + | <div class="block title"><h3 style="text-align: left;">Targeting pathogenic bacteria and Chassis</h3></div> |
<div class="block full"> | <div class="block full"> | ||
| − | <p>The first thing we needed to do was to narrow down the pathogenic bacteria we would work on. After researching in the literature and talking with experts in the fields of prosthetics, we found out that <i> | + | <p>The first thing we needed to do was to narrow down the pathogenic bacteria we would work on. After researching in the literature and talking with experts in the fields of prosthetics, we found out that <i>S. aureus</i> and <i>Staphylococcus epidermidis</i> are the two most frequently found bacterial infectious agents on implants. We decided to use the biobrick designed by the iGEM team SDU-Denmark 2009 who worked on a genetic circuit enabling <i> Escherichia coli </i> to actively detect <i> S. aureus </i>. </p> |
| − | <p>Our bacterial chassis of choice, which would inhibit the quorum sensing of <i> | + | <p>Our bacterial chassis of choice, which would inhibit the quorum sensing of <i> S. aureus</i>, needed to be a bacterium naturally occurring inside the human body. Indeed, we didn’t want to introduce a new strain inside the human body. The chassis also needed to have a good secretion system, as well as a good tolerance for the immune system. We narrowed down our choices between <i>Bacillus subtilis</i>, which the iGEM LMU Munich 2014 worked on, with the same problematic, and <i>E. coli</i>, naturally living inside the gastrointestinal tract of humans with mostly no problem except for some strains. Because we wanted to improve the construction of iGEM SDU Denmark 2009 and because of convenience inside our lab, since we already had competent <i>E. coli</i> cells, we decided to use this strain. We optimized our sequences for an <i>E. coli</i> expression, and adapted them to its secretion system by adding export signals. This way, our construction could be broadened and used in a different type of bacterium. To replicate our plasmids, we used <i>E. coli</i> DH5 alpha and to secrete our proteins, <i>E. coli</i> BL21De3 pLys S. </p> |
</div> | </div> | ||
<div class="block title"><h3 style="text-align: left;">Conceptualizing our Proof of Concept </h3></div> | <div class="block title"><h3 style="text-align: left;">Conceptualizing our Proof of Concept </h3></div> | ||
<div class="block full"> | <div class="block full"> | ||
| − | <p>When conceptualizing our proof of concept device, we decided to design a microfluidic chip to simulate the actions that would occur inside the patient’s body. The chip would be capable of measuring the neuronal signal as well as the conductivity of the biofilm, letting us know whether our system would work correctly inside a prosthetic. After talking with many professionals such as Dr. Heng Lu (ESPCI) and Dr Ayako Yamada (ENS, Ecole Normale Supérieure, Paris), we first designed a PDMS microfluidic chip with a vitreous carbon electrode to measure the signals. After talking with Dr. Catherine Villard (Institut Curie, Paris) and Dr. Frederic Khanoufi (University Paris Diderot—ITODYS), an electrochemist, we realized this type of electrode was highly sensitive, perhaps too sensitive for our type of device, and not adaptable to the size of our microfluidic chip. Guided by their advice, we switched to gold electrodes and started the fabrication process of the different type of chips at the Pierre Gilles de Gennes Institute. After attending the iCOE 2018, we learned about PEDOT (poly(3,4-ethylene dioxythiophene) polystyrene sulfonate ), and its conductive properties. As we also wanted to confine our bacteria so they would not harm our neuronal cells, we partnered with <a href="https://www.sterlitech.com">Sterlitech</a>, and tested nanoporous polycarbonate membranes coated in gold as well as nanoporous alumina oxide membrane coated in PEDOT: PSS, PEDOT: CL and PEDOT: TS. This way, we could still measure the neuronal signal and the conductivity of the biofilm while protecting the cells from getting eaten by the bacteria. </p> | + | <p>When conceptualizing our proof of concept device, we decided to design a microfluidic chip to simulate the actions that would occur inside the patient’s body. The chip would be capable of measuring the neuronal signal as well as the conductivity of the biofilm, letting us know whether our system would work correctly inside a prosthetic. After talking with many professionals such as Dr. Heng Lu (ESPCI) and Dr. Ayako Yamada (ENS, Ecole Normale Supérieure, Paris), we first designed a PDMS microfluidic chip with a vitreous carbon electrode to measure the signals. After talking with Dr. Catherine Villard (Institut Curie, Paris) and Dr. Frederic Khanoufi (University Paris Diderot—ITODYS), an electrochemist, we realized this type of electrode was highly sensitive, perhaps too sensitive for our type of device, and not adaptable to the size of our microfluidic chip. Guided by their advice, we switched to gold electrodes and started the fabrication process of the different type of chips at the Pierre Gilles de Gennes Institute. After attending the iCOE 2018, we learned about PEDOT (poly(3,4-ethylene dioxythiophene) polystyrene sulfonate ), and its conductive properties. As we also wanted to confine our bacteria so they would not harm our neuronal cells, we partnered with <a href="https://www.sterlitech.com"style="font-weight: bold ; color:#85196a;" target="__blank">Sterlitech</a>, and tested nanoporous polycarbonate membranes coated in gold as well as nanoporous alumina oxide membrane coated in PEDOT: PSS, PEDOT: CL and PEDOT: TS. This way, we could still measure the neuronal signal and the conductivity of the biofilm while protecting the cells from getting eaten by the bacteria. </p> |
</div> | </div> | ||
<div class="block title"><h3 style="text-align: left;">Designing the interface between the tissues and the prosthetis</h3></div> | <div class="block title"><h3 style="text-align: left;">Designing the interface between the tissues and the prosthetis</h3></div> | ||
<div class="block full"> | <div class="block full"> | ||
| − | <p>When we started to think about the scientific aspect of our project, we also started to design and think about how our biofilm would integrate into a physical medical device. First, we wanted to design a full prosthesis. We realized that it wasn’t the core of the problem. Indeed, the technology missing in this field was the actual interface between the prosthesis and the osseointegrated steel/titanium/ceramic stem inside the human body, limiting the field of bionic prosthesis. We decided to focus on this interface and started to talk with Dr. Benjamin Bouyer, a | + | <p>When we started to think about the scientific aspect of our project, we also started to design and think about how our biofilm would integrate into a physical medical device. First, we wanted to design a full prosthesis. We realized that it wasn’t the core of the problem. Indeed, the technology missing in this field was the actual interface between the prosthesis and the osseointegrated steel/titanium/ceramic stem inside the human body, limiting the field of the bionic prosthesis. We decided to focus on this interface and started to talk with Dr. Benjamin Bouyer, a lumbar rachis surgeon, on how we could deposit the biofilm on or in the osseointegrated stem. We realized that the stem would be in direct contact with the surgeon meaning that if we coated the whole structure with our biofilm, it would probably be stripped off, get deposited on the gloves and contaminate other areas. We also spoke with one member of the board of directors of ADEPA, “Association de Défense et d’Etude des Personnes Amputés », which translates to « Association for the Defense and Study of Amputated Persons” who is himself an amputee. He gave us great advice on the designing phase of this interface and raised the issue of the socket causing excessive sudation and discomfort for the patient. After meeting with experts from i.CERAM (Ceramic medical devices company, Limoges, France) and the CERAH (Center for Studies and Research on the Equipment for the Handicapped), we learned more and more about the different materials used in the making of prostheses. At this point, we realized that doing a full stainless-steel interface would stimulate the growth of bacteria in a biofilm structure. Therefore, we decided to switch and do the part in direct contact with the patient in ceramic, knowing that we still have the same problem that i.CERAM and the CERAH were facing currently. Indeed, steel doesn’t last as much as ceramic, which could be a problem when creating an interface composed of both materials. We knew then that the patient would probably need corrective surgeries to fix his osseointegrated stem. Having considered these parameters, we then decided to start modeling our prototype by integrating those different aspects as much as possible. Because we wanted it to be cost-effective and injection-moldable, we want to build our future prototypes in ABS, a thermoplastic polymer that could be molded by injection. We ordered the electronic parts composed of a charger, battery, and amplifier, and assembled it into its current state as our POC. </p> |
</div> | </div> | ||
<div class="block title"><h3 style="text-align: left;">Addressing law issues</h3></div> | <div class="block title"><h3 style="text-align: left;">Addressing law issues</h3></div> | ||
<div class="block full"> | <div class="block full"> | ||
| − | <p>While we were doing adjustments to our design in the lab, our team of jurists also researched how our device could be integrated into society given the current political and economic landscape in France. The use of GMOs, for the environment, food industry or medicine, is highly regulated in France and Europe. It is limited to design animal models of diseases and to produce large quantities of molecules for the pharmaceutical industry. The use of genetically modified bacteria inside the human body is not the subject of specific laws, which made our research on the subject difficult. We focused our research on medical device’s regulation in order to see if our project could be marketed. At first, it appeared almost naturally that our project was a medical device, but as it has to be understood as a legal term, it responds to a specific definition. Working on medical devices’ regulations helped us to better define our project, classify it and analyze our project in the scope of European regulations. We also tried to cover some legal questions related to our project and tried to find answers | + | <p>While we were doing adjustments to our design in the lab, our team of jurists also researched how our device could be integrated into society given the current political and economic landscape in France. The use of GMOs, for the environment, food industry or medicine, is highly regulated in France and Europe. It is limited to design animal models of diseases and to produce large quantities of molecules for the pharmaceutical industry. The use of genetically modified bacteria inside the human body is not the subject of specific laws, which made our research on the subject difficult. We focused our research on the medical device’s regulation in order to see if our project could be marketed. At first, it appeared almost naturally that our project was a medical device, but as it has to be understood as a legal term, it responds to a specific definition. Working on medical devices’ regulations helped us to better define our project, classify it and analyze our project in the scope of European regulations. We also tried to cover some legal questions related to our project and tried to find answers from other legal systems such as the American one.</p> |
</div> | </div> | ||
| Line 83: | Line 92: | ||
<div class="block full bothContent"> | <div class="block full bothContent"> | ||
<div class="block dropDown" id="Silver"> | <div class="block dropDown" id="Silver"> | ||
| − | <h4>Silver</h4> | + | <h4>Silver: Our Project in Society</h4> |
</div> | </div> | ||
<div class="block hiddenContent"> | <div class="block hiddenContent"> | ||
<span class="closeCross"><img src="https://static.igem.org/mediawiki/2018/6/67/T--Pasteur_Paris--CloseCross.svg"></span> | <span class="closeCross"><img src="https://static.igem.org/mediawiki/2018/6/67/T--Pasteur_Paris--CloseCross.svg"></span> | ||
<div class="block title"> | <div class="block title"> | ||
| − | |||
<p><i>One of the main concerns we had while developing our synthetic biology project was to determine in which way our biofilm could be used and who would be using it, in order to evaluate its impact on society. The field which would benefit the most of a nerves redirection while reducing the risk of infection, was orthopedic surgery, targeting patients suffering amputation.</i></p> | <p><i>One of the main concerns we had while developing our synthetic biology project was to determine in which way our biofilm could be used and who would be using it, in order to evaluate its impact on society. The field which would benefit the most of a nerves redirection while reducing the risk of infection, was orthopedic surgery, targeting patients suffering amputation.</i></p> | ||
</div> | </div> | ||
| + | <div class="block title"><h1>Investigating Infection During Surgery</h1></div> | ||
| + | <div class="block full"> | ||
| + | <p>In medicine, surgery is a very common procedure. According to the data available, gathered by the Royal College of Surgeons, around 4.7 million surgical procedures were performed in 2014 in England; meaning that 7% of the total population is impacted by surgery. In the United States of America, according again to data gathered by the 2010 Ambulatory Medical Care Survey (NHAMCD), 48.23 million surgical procedures were performed in 2010. That’s around 14.8% of the total population. 5% of patients undergoing surgery develop surgical site infections (SSIs), which may cause substantial morbidity that can endanger a patient’s life, increase the number of days in the hospital and increase healthcare costs. SSIs are defined as infections that occur within 1 year if an implant is being placed.</p> | ||
| + | <p>We wanted to understand what goes on during a surgical procedure, what types of precautions were taken and what were the impacts of an infection for the patient. Thus, we began contacting surgeons and associations to learn more. </p> | ||
| + | </div> | ||
| + | <div class="block title"><h3 style="text-align: left;">European Hospital Georges-Pompidou (HEGP) in Paris </h3></div> | ||
| + | <div class="block two-third"> | ||
| + | <p>The European Hospital Georges-Pompidou is the newest and biggest Parisian hospital. It opened in 2001, receiving the name of one of the French Presidents, Georges Pompidou. It’s one of the most efficient hospitals in Europe, illustrating itself by being the first cardiac transplant facility on the continent. </p> | ||
| + | <p>At the HEGP, we learned about the methods used to minimize the risks of infection, as well as how the surgeon was approaching those risks for the patients. With authorization from Dr. Benjamin Bouyer, we also had the chance to observe a team of surgeons during a spine implant operation. It became clear to us that even though every step was taken to eliminate the risk of infection, it could be useful to develop a system to minimize one step further this risk in the case of an implant. </p> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/1/16/T--Pasteur_Paris--Logo-HEPG.png"> | ||
| + | </div> | ||
| + | <div class="block two-third"> | ||
| + | <h4 style="text-align: left;">Interview with Dr. Benjamin Bouyer </h4> | ||
| + | <p>Dr. Benjamin Bouyer’s main concern with our project was how we would be incorporating the biofilm inside the patient. Indeed, every implant is given to the surgeon in a sterile way, inside a bag the doctor would not open until the very last moment. He also brought up the issue of our system being 3 in 1. Indeed, our biofilm would be capable of reducing the risk of infection by Staphylococcus aureus but would also be modified to induce the growth of nerves when needed, all this while conducting a signal. Indeed, our project is focusing on amputated patients, meaning only they would need all the three branches of our system. We reassured him by explaining that our system could also be used only for its inhibition of virulence properties and that it could be open to every type of patient. He did, however, believe that our project could be useful to many branches of medicine. We also asked him if he would, as a surgeon, use our device if the laws changed about the use of GMOs, and his response was very positive. Indeed, in his opinion, no doctor would reject a system proven to reduce infections. </p> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/7/77/T--Pasteur_Paris--DrBenjaminBouyer.jpg" style="max-width: 350px;"> | ||
| + | <p><i>Dr. Benjamin Bouyer is a surgeon at the HEGP specialized in lumbar rachis surgeries. He is currently clinical assistant head at the hospital and is also part of the “Société Française de Chirurgie du Rachis” or in english the “French Society of Rachis Surgeries”. </i></p> | ||
| + | </div> | ||
| + | <div class="block title"><h3 style="text-align: left;">The Institut Pasteur</h3></div> | ||
| + | <div class="block one-third"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/e/e6/T--Pasteur_Paris--Ghigo.png"> | ||
| + | <p><i>Pr. Jean-Marc Ghigo is the head of the Genetics of Biofilms Unit at Institut Pasteur. He is interested in the mechanisms involved in infections independent of a material, and infection linked to an implanted material. </i></p> | ||
| + | </div> | ||
| + | <div class="block two-third"> | ||
| + | <h4 style="text-align: left;">Interview with Pr. Jean-Marc Ghigo.</h4> | ||
| + | <p>Pr. Jean-Marc Ghigo was the first professional to talk to us about the formation of biofilms. In addition to his help with lab biofilm growth protocols, he also gave us great advice concerning our device and the confinement of our biofilm. Indeed, biofilms have a tendency to colonize the system and we needed to think of a way to confine them so they don’t disperse in the patient’s body or contaminate other material during the integration of the implant. By confining the biofilm, we would also reduce the chances of bacterial conjugation between possible pathogenic bacteria and ours. </p> | ||
| + | </div> | ||
| + | <div class="block separator-mark"></div> | ||
| + | |||
| + | <div class="block title"><h1>Investigating Neural Connections and Prostheses</h1></div> | ||
| + | |||
| + | <div class="block title"><h3 style="text-align: left;">ICM</h3></div> | ||
| + | <div class="block two-third"> | ||
| + | <p>The “Institut du Cerveau et de la Moelle épinière” - ICM (Brain and Spine Institute), is an international brain and spinal cord research center in Paris whose innovative concept and structure make it the only institute of its kind in the world. The ICM brings patients, doctors, and researchers together with the aim to develop treatments for disorders of the nervous system and enable patients to benefit from them as quickly as possible. </p> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <a href= "https://icm-institute.org/en/"><img src="https://static.igem.org/mediawiki/2018/7/77/T--Pasteur_Paris--ICM.png"></a> | ||
| + | </div> | ||
| + | <div class="block full"> | ||
| + | <h4 style="text-align: left;">Interview with Dr. Bernard Zalc, Ph.D</h4> | ||
| + | <p><i>Dr. Bernard Zalc is co-leader of the oligodendrocyte development and neurovascular interactions department. He studies oligodendroglial cell development in the embryonic brain and the interactions between neural cells and the cerebral vascular network. </i></p> | ||
| + | <p> | ||
| + | Dr. Bernard Zalc facilitated our understanding of the nerve growth and explained to us some issues that we could encounter. He gave us some tips regarding nerve growth and the connection between the nerve and our interface. Indeed, for him, other neurotrophins (like BDNF and VEGFA) and chemoattractant molecules are needed in order to attract and direct the growth toward the implanted interface of our system. We exposed to him our experimental approach for the proof of concept and he talked about the importance of the Schwann cells and the myelinization for the conduction. Moreover, he shared contacts which can help us regarding the modeling parts and the prosthesis. | ||
| + | </p> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | <div class="block title"><h3 style="text-align: left;">SORBONNE UNIVERSITÉ</h3></div> | ||
| + | <div class="block two-third"> | ||
| + | <p>Sorbonne University is a multidisciplinary, research-intensive and world-class academic institution. Firmly rooted in the heart of Paris, it is committed to the success of its students and devoted to meeting the scientific challenges of the 21st century. </p> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <a href= "https://www.sorbonne-universite.fr/en"><img src="https://static.igem.org/mediawiki/2018/5/52/T--Sorbonne_U_Paris--LOGO_SCIENCES_SU_med.jpg"></a> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/d/d1/T--Pasteur_Paris--bisbis.jpg"> | ||
| + | <p><i>Dr. Jean-Marc Victor is a researcher at Sorbonne University. He is currently Director of Research at the CNRS, the French National Center for Scientific Research and is also working for the group “Multiscale Modeling of Living Matter at the Laboratory of Theoretical Physics of Condensed Matter. </i></p> | ||
| + | </br></div> | ||
| + | <div class="block two-third"> | ||
| + | <h4 style="text-align: left;">Interview with Jean-Marc Victor, Ph.D. </h4> | ||
| + | <p>Dr. Jean-Marc Victor was a real help for our modeling team. After presenting him the project, that he was very fond of, he helped us with the modeling of the growth of the neurons when we faced problems. His dynamism and interest for our project really helped us to finish our modeling, and in consequence, our in vitro primary culture. </p> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | <div class="block title"><h3 style="text-align: left;">ADEPA</h3></div> | ||
| + | <div class="block two-third"> | ||
| + | <p>ADEPA stands for “Association de Défense et d’Etude des Personnes Amputées », which translates to « Association for the Defense and Study of Amputated Persons ». This national association was created in 1996 and aims to unite forces between disabled persons. They represent the community during ministerial commissions and try to help people in their daily lives with their handicap by giving support and finding solutions. </p> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <a href= "https://www.adepa.fr"><img src="https://static.igem.org/mediawiki/2018/f/fb/T--Pasteur_Paris--Logo-ADEPA.png"></a> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/2/2c/T--Pasteur_Paris--JPHonsOlivierPortrait.jpg"> | ||
| + | <p><i>Jean-Pascal Hons-Olivier is a country security Manager and member of the board of directors of ADEPA. As a leg amputee who underwent multiple corrective surgeries on his leg, Mr. Hons-Olivier is very involved in ADEPA. </i></p> | ||
| + | </br> | ||
| + | </div> | ||
| + | <div class="block two-third"> | ||
| + | <h4 style="text-align: left;">Interview with Jean-Pascal Hons-Olivier </h4> | ||
| + | <p>Mr. Jean-Pascal Hons-Olivier gave us great input on the different causes of amputation and the relationship between a patient and his prosthetist. He also allowed us to understand what was the process of getting an implant in France, how someone is living with an amputated limb and also gave his opinion on the different type of prosthesis on the market. He also talked about the type of prosthesis our system would integrate into: osseointegrated prostheses. The type of surgery is currently not taken in charge by the French health care system due to the high risk of infection, and breaking of the bone and the prosthesis, and only one surgeon is doing this type of operation in the country: Dr. Marion Bernard. Mr. Hons-Olivier did raise up some concerns about our project being too innovative since only a few industries in the USA are working on bionic prostheses and this type of surgery costs more around 100 000 euros at the moment. </p> | ||
| + | </div> | ||
| + | <div class="block two-third center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/d/de/T--Pasteur_Paris--JPHonsOlivierRecadre.jpg"> | ||
| + | </div> | ||
| + | <div class="block title"><h3 style="text-align: left;">CERAH</h3></div> | ||
| + | <div class="block two-third"> | ||
| + | <p>Thanks to Jean-Pascal Hons-Olivier’s generosity and his prosthetist, Didier Azoulay, we visited CERAH, the center of studies and research on equipment for disabled persons. We attended a course on prostheses with students in physical therapy. Also, we had the opportunity to see a step of prosthesis fabrication: namely stump molding for its sleeve. During this afternoon, we discovered osseointegration as a surgical technique to implant the prosthesis. Most of the time, the arm or leg’s prostheses aren’t integrated into the body but just attached to the body by a silicon sleeve which maintains the device by creating the vacuum. We understood that this method causes an excessive transpiration due to the sleeve and can generate pain on the bone’s end. Encouraged by these observations and due to the fact that we need a physical link between the body’s inside and the prosthesis, we chose osseointegration to NeuronArch project.</p> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/1/1b/T--Pasteur_Paris--CERAH3.jpg"> | ||
| + | <div class="legend"></div> | ||
| + | </div> | ||
| + | <div class="block title"><h3 style="text-align: left;">i.CERAM </h3></div> | ||
| + | <div class="block two-third"> | ||
| + | <p>i.CERAM based in Limoges, France, was founded in 2005 and is designing, manufacturing and marketing high-tech implants for various joints of the human body. The marriage of different ceramic materials and processes is one of the main features of the company. Thus, the clinical experience, combined with compressive strength qualities, osseocompatibility or decreased friction ceramics are exploited in the design of new implants of the company. The best seller of the company is a ceramic implant charged in antibiotics. </p> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <a href="http://www.iceram.fr/en/"><img src="https://static.igem.org/mediawiki/2018/2/28/T--Pasteur_Paris--Logo-ICeram.png"></a> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/e/e3/T--Pasteur_Paris--iceram2.png"> | ||
| + | <div class="legend">Porous Ceramic</div> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/9/92/T--Pasteur_Paris--iceram3.png"> | ||
| + | <div class="legend">Prosthesis made of polished ceramic</div> | ||
| + | </div> | ||
| + | <div class="block full"> | ||
| + | <h4 style="text-align: left;">Interview with Dr. Eric DENES, Dr. Evelyn Poli and Dr. Christelle Arico </h4> | ||
| + | <p><i>Dr. Eric Denes is an infectious diseases specialist at the University Hospital of Limoges and is the scientific director of i.CERAM. Dr. Evelyn Poli is a chemistry research and development engineer and Dr. Christelle Arico is a project manager at i.CERAM. </i></p> | ||
| + | </br> | ||
| + | <p>Dr. Eric Denes, as well as Dr. Evelyn Poli and Dr. Christelle, were very interested in our project. They are currently designing new materials in ceramic, titanium, and stainless-steel to test the bacterial adhesion on their prostheses. They warned us about the use of metals for the prostheses since <i>S. aureus</i> and <i>P. aeruginosa</i> tend to form biofilms easily on metals rather than on ceramic. They are also using Gentamicin and Vancomycin charged implants to cure infections. Their system does have a long half-life time (4 days) whereas our biofilm could be a longer-term solution. </p> | ||
| + | </div> | ||
| + | <div class="block title"> | ||
| + | <h3 style="text-align: left;">IRAMIS </h3> | ||
| + | </div> | ||
| + | <div class="block full"> | ||
| + | <p>IRAMIS is at the Saclay Institute of Matter and Radiation and is the second institute in size of the CEA Fundamental Research Division. Inside IRAMIS there are projects directly linked to nanoscience for the technology of information and health, the interaction between matter, radiation and low-carbon energy applications. </p> | ||
| + | </div> | ||
| + | <div class="block two-third"> | ||
| + | <h4 style="text-align: left;">Interview with Dr. Bernard Geffroy</h4> | ||
| + | <p>We contacted Dr. Bernard Geffroy to learn more about OLED and to see if we could integrate the technology he is currently working on inside the prosthesis. It could be a way to let the patient know if S. aureus is present at the junction site of its osseointegrated prosthesis. Dr. Bernard Geffroy did go along the sayings of Mr. Jean-Pascal Hons Olivier on our project, mentioning it as being a bit innovative for now on. At first, we wanted to know if he thought the integration of a flexible screen inside the prosthesis, letting the patient know at every moment his/her health data, was possible. Dr. Bernard Geffroy did applaud for our ideas but doesn’t think this technology would be on the market for at least 10 or 20 years. He did redirect our ideas towards the use of OLEDs and color codes and was really interested in the following of our project. </p> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/a/a1/T--Pasteur_Paris--iramis.png"> | ||
| + | <p><i>Dr. Bernard Geffroy is currently working at the IRAMIS on OLED and flexible panels technology. OLED stands for Organic Light-Emitting Diode, in which the emissive electroluminescent layer is a film of organic compound that emits light in response to an electric current. </i></p> | ||
| + | </div> | ||
| + | <div class="block title"><h3 style="text-align: left;">PARIS CITY HALL (Mairie de Paris)</h3></div> | ||
| + | <div class="block two-third"> | ||
| + | <p>The Paris City Hall is the headquarters of municipal and departmental representatives. In 2024, after 100 years, Paris will be hosting once again the Olympic games and Paralympic games. The city hall has 3 priorities toward this event. First, to create a more just society for everyone, where nobody is left aside. Second, a better participation and social integration of handicapped people. Third, to increase the spectator’s and partner’s interest for the Paralympic games.</p> | ||
| + | </div> | ||
| + | <div class="block one-third"> | ||
| + | <a href="https://www.paris.fr"><img src="https://static.igem.org/mediawiki/2018/d/d0/T--Pasteur_Paris--LogoMairiedeParis.png"></a> | ||
| + | </div> | ||
| + | <div class="block full"> | ||
| + | <h4 style="text-align: left;">Contact with Cyril Cartron and Marion Liard</h4> | ||
| + | <p><i>Cyril Cartron and Marion Liard are two members of the Paris City Hall. Cyril Cartron works for the DICOM (Direction of Information and Communication) and deals with all events organized by the City Hall, especially sporting events. Marion Liard is a project manager in the Town Communication department. Her actions are particularly devoted to handicap. </i></p> | ||
| + | </br> | ||
| + | <p>Since NeuronArch is a project directly linked to handicap, we decided to get involved with the Mairie de Paris to make Paris a more accessible town for disabled people by creating new markings and signs in direct connection with the Paralympic games. For this, we contacted the Paris City Hall to make this project in collaboration, especially in preparation for 2024 Paralympics in Paris. </p> | ||
| + | <p>First, we contacted Cyril Cartron by phone. He was determined to help us. He gave us the contact of Marion Liard, in charge of handicap events. After some emails, she put us in contact with the cabinet of Nicolas Nordman, deputy mayor of the Paris City Hall, responsible for all issues concerning disabled people and their accessibility. This cabinet will contact us for a conciliation meeting, the December 3rd, to discuss with specialists during the International Handicap Day. Thus, we are eager to do this meeting with them! </p> | ||
| + | </div> | ||
| + | |||
</div> | </div> | ||
</div> | </div> | ||
| Line 98: | Line 250: | ||
<div class="block dropDown" id="Survey"> | <div class="block dropDown" id="Survey"> | ||
<h4>Survey</h4> | <h4>Survey</h4> | ||
| − | + | </div> | |
<div class="block hiddenContent"> | <div class="block hiddenContent"> | ||
<span class="closeCross"><img src="https://static.igem.org/mediawiki/2018/6/67/T--Pasteur_Paris--CloseCross.svg"></span> | <span class="closeCross"><img src="https://static.igem.org/mediawiki/2018/6/67/T--Pasteur_Paris--CloseCross.svg"></span> | ||
<div class="block title"> | <div class="block title"> | ||
| + | |||
| + | <h1>Introduction</h1></div> | ||
| + | <div class="block full"> | ||
| + | <p>The project NeuronArch is also based on a survey that gives us information to orient, improve and integrate our ideas. This survey has three main goals. They concern both our project and synthetic biology in general: </p> | ||
| + | <p> <b> | ||
| + | </br> | ||
| + | - Vulgarize synthetic biology | ||
| + | </br> | ||
| + | - Introduce our project to the general public | ||
| + | </br> | ||
| + | - Validate our project by health professionals | ||
| + | </br> | ||
| + | </b></p> | ||
| + | |||
| + | <p> To achieve those goals, we had to think about the survey’s framework. For that, we decided to categorize two population types: the general public (children, students, parents…) and health professionals. Then, we conceived a map of our survey which is connected to our goals and our different target populations. </p> | ||
| + | |||
| + | <img src="https://static.igem.org/mediawiki/2018/8/8c/T--Pasteur_Paris--Survey_Framework.png"> | ||
| + | <div class="legend"><b>Figure 1:</b> The framework of our survey</div> | ||
| + | |||
| + | <p> As shown by the map above, there are <b> two common sections </b>: the first part includes general questions, synthetic biology, and biofilms for all, and the second part to collect feedback and opinions. Then, <b> a specific section about infections aimed at health professional </b> in the field of prostheses. This choice enabled us to collect relevant information concerning the project NeuronArch and to achieve the third goal of this survey. | ||
| + | |||
| + | Besides the distinction between general public and medical professionals, we wanted to differentiate <b> their various levels in biology </b>. Therefore, we asked a question about their science education level. This question helped us to analyze answers about synthetic biology for example. | ||
| + | |||
| + | <i> After defining goals, different populations and framework of our survey, we had to think on how to analyze the results. </i> </p> | ||
| + | </div> | ||
| + | |||
| + | <div class="block title"> | ||
| + | <h1>Approach through trial and error</h1></div> | ||
| + | <div class="block half"> | ||
| + | <p> This approach was adopted just after collecting our first answers. The last section of the survey is dedicated to public’s opinion and feedback about the survey. With this section, we can gather all criticism and advice to improve the survey (and the project too!). </p> | ||
| + | <img src= "https://static.igem.org/mediawiki/2018/f/f1/T--Pasteur_Paris--SurveyApproachBis.png"> | ||
| + | <div class="legend"><b>Figure 2:</b> Our survey methodology</div> | ||
| + | </div> | ||
| + | <div class="block full"> | ||
| + | <p> As a consequence, we adapted our explanation section making it more readable. | ||
| + | We added some questions: for example, we decided to ask biology levels to improve our analysis and to better understand comments and feedback. | ||
| + | We also modified some questions: | ||
| + | </br> | ||
| + | - About the age range | ||
| + | </br> | ||
| + | - About the infection rate in prosthesis: we discussed among ourselves and took into account comments that mentioned that the question was too confusing. | ||
| + | |||
| + | Moreover, we received other comments about the project and the scientific part. Following a suggestion in the comments, we thought about developing a collaborative approach allowing the public to get an answer to their questions, and hence make it more interactive. This is an aspect that we will consider in the future. | ||
| + | |||
| + | This process through trial and error enabled us to improve the survey and obtain relevant answers. </p> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | <div class="block title"> | ||
| + | <h1>Data analysis</h1></div> | ||
| + | <div class= "block full"> <p> See all our data in this <a href="https://static.igem.org/mediawiki/2018/6/6d/T--Pasteur_Paris--Survey_Answers.xlsx"style="font-weight: bold ; color:#85196a;" target="__blank">Excel file.</a></p></div> | ||
| + | <div class="block title"> | ||
| + | <h2>Population</h2></div> | ||
| + | </br> | ||
| + | <div class="block full"> | ||
| + | <p> Our survey was launched on August 21st, 2018 on the platform Google Form and was destined to the French public. We received 205 answers from 80 men and 125 women.</p></div> | ||
| + | <div class= "block two-third center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/e/e6/T--Pasteur_Paris--Survey_Gender.png"> | ||
| + | <div class="legend"><b>Figure 3:</b> Population gender of our study</div> </div> | ||
| + | |||
| + | <div class="block half"> | ||
| + | <p>We tried to diffuse our survey on the internet, trying to reach as many people as possible. At the beginning we noticed that most of the answers were from the younger people, between the ages of 16 and 25, almost all in the same age range as our team members ! We then tried to reach a wider population, from the ages of 26 and above 71.</p></div> | ||
| + | <div class="block half"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/9/9d/T--Pasteur_Paris--Survey_Age.png"> | ||
| + | <div class="legend"><b>Figure 4:</b>The surveyed population's age ranges</div></div> | ||
| + | |||
| + | |||
| + | <div class="block full"> | ||
| + | <p>In the end, although we still have a majority of young people between 16 and 25. However, we had an improvement of almost 1/3 of the answers coming from other age ranges. | ||
| + | </p></div> | ||
| + | <div class="block half"> | ||
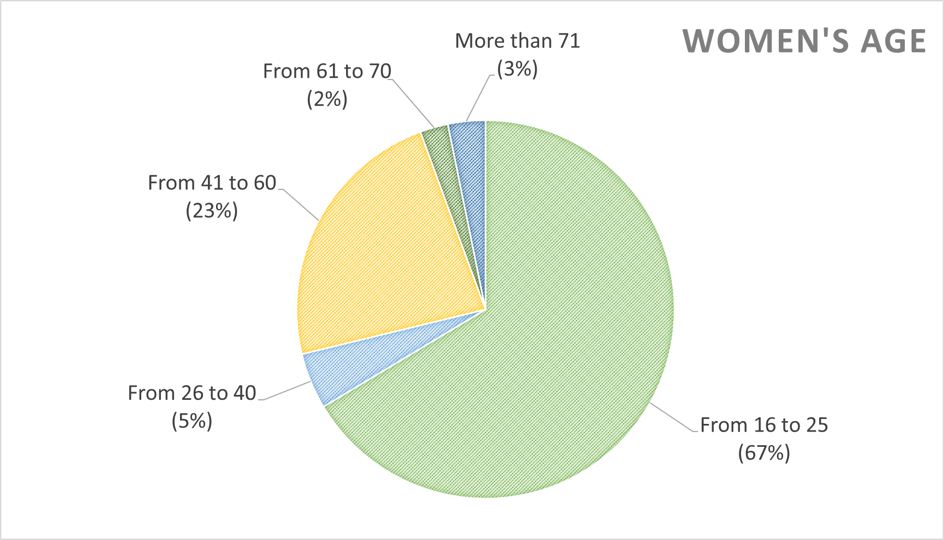
| + | <img src="https://static.igem.org/mediawiki/2018/b/b4/T--Pasteur_Paris--Survey_WomenAge.png"> | ||
| + | <div class="legend"><b>Figure 5:</b> Zoom on women's age</div></div> | ||
| + | <div class="block half"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/3/3c/T--Pasteur_Paris--Survey_MenAge.png"> | ||
| + | <div class="legend"><b>Figure 6:</b> Zoom on men's age</div></div> | ||
| + | |||
| + | <div class="block full"> | ||
| + | <p>With the first version of our survey, we did not ask details about the scientific background, and especially the background in biology. However, we quickly realized while reading some comments and seeing the first results that feedback on the survey, and on what people understood or already knew, was highly related to their background in biology. | ||
| + | For example, we were surprised at some of the first people who answered saying that the survey did not help them to better understand synthetic biology but with this question about the background in biology, we discovered that some of them had a Ph.D. or were College students in biology and therefore did not get any new information from our explanation. | ||
| + | </p></div> | ||
| + | <div class="block two-third center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/2/2f/T--Pasteur_Paris--Survey_Backgroundbiology.png"> | ||
| + | <div class="legend"><b>Figure 7:</b> Backgrounds in biology of the surveyed population</div></div> | ||
| + | |||
| + | |||
| + | <div class ="block full"> | ||
| + | <div class="block title"> | ||
| + | <h2>Opinion on synthetic biology</h2></div> | ||
| + | </br> | ||
| + | <div class="block full"> | ||
| + | <p> One of the goals of our survey was to open up synthetic biology to a wider population by asking questions on it and explaining what it is and what it is for. | ||
| + | We wanted to know whether people knew about it and whether they had any interest in it. We can see from Figure 8 that the majority of people are interested in synthetic biology or GMOs. Although, 6% seem not to be interested. </p></div> | ||
| + | <div class ="block two-third center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/f/f0/T--Pasteur_Paris--Survey_GMO.png"> | ||
| + | <div class="legend"><b>Figure 8:</b> Opinions on GMOs</div></div> | ||
| + | |||
| + | <div class="block full"> | ||
| + | <p>We can also observe that 100% of those polled have a good opinion about synthetic biology’s utility. Indeed, even though 75% of those polled thinks synthetic biology needs an ethical framework, it is still a field of study that could have a great role in driving science forward. </p></div> | ||
| + | |||
| + | <div class="block two-third center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/0/06/T--Pasteur_Paris--Survey_SyntheticBiologyInterest.png"> | ||
| + | <div class="legend"><b>Figure 9:</b> Interest of Synthetic Biology</div></div> | ||
| + | |||
| + | </br> | ||
| + | </br> | ||
| + | |||
| + | <div class="block half"> | ||
| + | <p>In general, some apprehensions still persisted.</p></div> | ||
| + | <div class="block half"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/4/42/T--Pasteur_Paris--Survey_SyntheticBiologyApprehension.png"> | ||
| + | <div class="legend"><b>Figure 10:</b> Apprehension about synthetic biology</div></div> | ||
| + | |||
| + | <div class ="block full"> | ||
| + | <div class="block title"> | ||
| + | <h2>Opinion on our project</h2></div> | ||
| + | </br> | ||
| + | <p>As our product is meant to be a medical device and inserted in the human body, an important question would be: would people accept to wear our prosthesis? | ||
| + | We wanted to have the most honest answer to this question, therefore, we paid attention to the way we asked the question. The way we put it, therefore, was « If necessary and if a NeuronArch prosthesis were available on the market, would you be ready to integrate it to your body, or if they were in a category of health professionals, would they recommend such a medical device, integrating genetically modified organisms? ». | ||
| + | We were surprised by the rate a positive answer to this question. Indeed, only 11% of those polled answered they would not accept to wear our device. Among health professionals, 36,5% would recommend our prosthesis to their patients, whereas 54,5% do not know and about 9% would not recommend our device. It is important to point out the fact that the same percentages are found concerning their answers to the question "would you wear a Neuronarch prosthesis?". </p></div> | ||
| + | <div class ="block two-third center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/0/03/T--Pasteur_Paris--Survey_WearNeuronArch.png"> | ||
| + | <div class="legend"><b>Figure 11:</b>Percentage of people who would accept to wear NeuronArch prosthesis </div></div> | ||
| + | |||
| + | <div class="block full"> | ||
| + | <p>Our survey was also a way to present our project to health professionals. | ||
| + | Even though this part was hard to fill, as it requires 2 characteristics (working in the field of health; being in contact with amputees), it was very interesting to analyze the answers of persons working in the medical fields and to read their comments. We discovered that there was a real interest in our project. | ||
| + | For example, we asked whether they thought our project was feasible and what they were thinking of our project. Out of 8 answers, 6 were « Yes », 1 was « Maybe » and the last was a comment on our project « Project that tries to improve a real current problem ». | ||
| + | We also gathered some information on the percentage of implant-related infections, the ways to improve the comfort of the patients who are wearing a prosthesis and information on how implant-related infections are treated. </p></div> | ||
| + | <div class="block two-third center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/e/e7/T--Pasteur_Paris--Survey_WaysToTreat.png"> | ||
| + | <div class="legend"><b>Figure 12:</b> Answers about ways to treat infections</div></div> | ||
| + | |||
| + | |||
| + | <div class="block full"> | ||
| + | <div class="block title"> | ||
| + | <h2>Feedback on our survey</h2></div> | ||
| + | </br> | ||
| + | <p> The last part of our survey was a feedback on itself. We wanted to know if it had helped people to understand what synthetic biology is and if our explanations were sufficient. | ||
| + | We asked questions about whether or not our survey helped them understand what synthetic biology is, on the clarity of our explanations and the relevance of our questions. | ||
| + | In order to better evaluate the impact of our survey, we wanted people to evaluate their knowledge of synthetic biology before and after answering our survey. </p></div> | ||
| + | <div class="block two-third center"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/d/d0/T--Pasteur_Paris--Survey_FeedbackSyntheticBiology.png"> | ||
| + | <div class="legend"><b>Figure 13:</b> Feedback on synthetic biology</div></div> | ||
| + | |||
| + | |||
| + | <div class="block half"> | ||
| + | <p> One of the most important aspect of this study is that, among those who did not know synthetic biology at all (14%), more than the half have a positive evaluation of their knowledge of synthetic biology. Indeed, to the question: <i>Did our survey help you to better understand what synthetic biology is?</i> they gave 4/5 or 5/5 as a grade.</br> | ||
| + | Concerning the clarity of our explanations, we asked people to rate it, thanks to a scale going from 1 to 5 (1: explanations lack clarity; 5: explanations are very clear).</p></div> | ||
| + | <div class="block half"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/5/52/T--Pasteur_Paris--Survey_Clarity.png"> | ||
| + | <div class="legend"><b>Figure 14:</b> Clarity of our survey</div></div> | ||
| + | |||
| + | |||
| + | |||
| + | <div class="block half"> | ||
| + | <p>We also asked people to explain their answers. Three main types of comments stood out: on one hand, people said that our survey was clear and well explained with a good point for illustrations and photographs, but on the other hand, it also appeared that it was too long and repetitive. It also appears for 6 people that our survey was not vulgarized enough and was difficult to understand when having no background in biology. </p></div> | ||
| + | <div class="block half"> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/8/87/T--Pasteur_Paris--Survey_SampleComments.png"> | ||
| + | <div class="legend"><b>Figure 15:</b> Some comments about our survey</div></div> | ||
| + | <div class="block full"><p>Some comments: | ||
| + | </br> | ||
| + | « Je suis de tout coeur avec vous dans ce projet ! » (<i>“I am with you on this project with all my heart!”</i>) | ||
| + | </br> | ||
| + | « J'ai appris beaucoup d'informations sur le sujet que je ne connaissais pas » (<i>“I have learned a lot on this subject which I did not know before”</i>) | ||
| + | </br> | ||
| + | « Merci de m'avoir fait connaître votre projet et j'espère qu'il sera couronné de succès et qu'il pourra servir dans d'autres circonstances (éviter la multirésistance de certains germes, proposer d'autres thérapies ...) » (<i>“Thanks for making me discover your project and I hope it will be successful and that it will be useful in other circumstances (avoid multiresistance to some germs, offer other kinds of therapies…)”</i>) | ||
| + | </br> | ||
| + | « Sujet intéressant, qui pourra, et j'espère améliorer la vie des personnes amputées. Et surtout bon courage et bonne chance à vous. » (“<i>Interesting subject, that will, and I hope, improve the life of amputees. Above all good luck</i>”)</p> | ||
| + | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
Latest revision as of 14:29, 10 November 2018