| Line 27: | Line 27: | ||
<p style="font-size:18px"> The experiment 1 illustrates the relationship between the concentration of glucose and the absorbance of the solution with DNS colorant. By using linear regression, we constructed a function with glucose concentration (μmol) as the x-axis and the ab-sorbance as the y-axis. </p> | <p style="font-size:18px"> The experiment 1 illustrates the relationship between the concentration of glucose and the absorbance of the solution with DNS colorant. By using linear regression, we constructed a function with glucose concentration (μmol) as the x-axis and the ab-sorbance as the y-axis. </p> | ||
| − | <p>In the experiment 2, we measured the stability of sucrose and dextran, as well as pH stability. We let the reaction take place in the hot pot set at 100℃. We observed that dextran has a poor hot stability. Moreover, the sucrose will decompose spontaneous-ly under the acidic condition while heat doesn’t affect the decomposition of sucrose. | + | <p style="font-size:18px">In the experiment 2, we measured the stability of sucrose and dextran, as well as pH stability. We let the reaction take place in the hot pot set at 100℃. We observed that dextran has a poor hot stability. Moreover, the sucrose will decompose spontaneous-ly under the acidic condition while heat doesn’t affect the decomposition of sucrose. |
</p> | </p> | ||
Latest revision as of 01:13, 18 October 2018


Experiment Data


Analysis
The experiment 1 illustrates the relationship between the concentration of glucose and the absorbance of the solution with DNS colorant. By using linear regression, we constructed a function with glucose concentration (μmol) as the x-axis and the ab-sorbance as the y-axis.
In the experiment 2, we measured the stability of sucrose and dextran, as well as pH stability. We let the reaction take place in the hot pot set at 100℃. We observed that dextran has a poor hot stability. Moreover, the sucrose will decompose spontaneous-ly under the acidic condition while heat doesn’t affect the decomposition of sucrose.
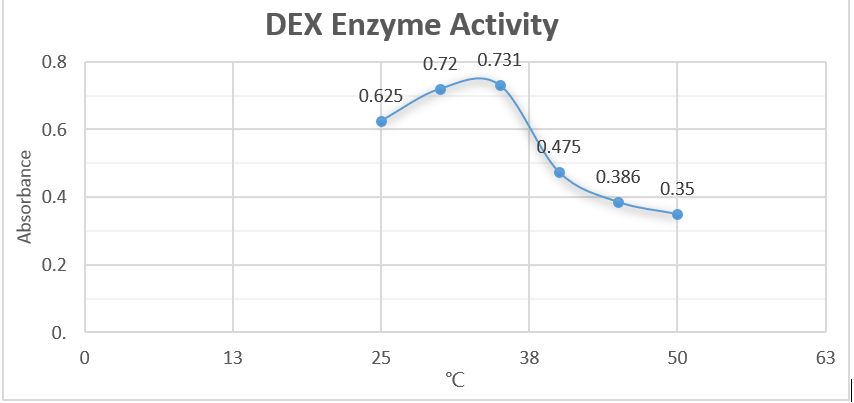
Analysis
From the first half of the graph, we can clearly discover the linear relationship be-tween enzyme concentration and the absorbance. Data suggest that as the concentra-tion of enzyme becomes greater, the relative absorbance increase proportionately. This result justified our assumption. However, the latter half illustrated a negative-related relationship. We suspected that we didn’t use enough substrate to allow the decomposition reaction. Also, we assumed that excessive concentration of enzymes will negatively affected the reaction rate.

File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 01:09, 18 October 2018 |  | 827 × 465 (528 KB) | Typing (Talk | contribs) |
File usage
There are no pages that link to this file.
