| Line 27: | Line 27: | ||
<p style="font-size:18px"> The experiment 1 illustrates the relationship between the concentration of glucose and the absorbance of the solution with DNS colorant. By using linear regression, we constructed a function with glucose concentration (μmol) as the x-axis and the ab-sorbance as the y-axis. </p> | <p style="font-size:18px"> The experiment 1 illustrates the relationship between the concentration of glucose and the absorbance of the solution with DNS colorant. By using linear regression, we constructed a function with glucose concentration (μmol) as the x-axis and the ab-sorbance as the y-axis. </p> | ||
| − | <p>In the experiment 2, we measured the stability of sucrose and dextran, as well as pH stability. We let the reaction take place in the hot pot set at 100℃. We observed that dextran has a poor hot stability. Moreover, the sucrose will decompose spontaneous-ly under the acidic condition while heat doesn’t affect the decomposition of sucrose. | + | <p style="font-size:18px">In the experiment 2, we measured the stability of sucrose and dextran, as well as pH stability. We let the reaction take place in the hot pot set at 100℃. We observed that dextran has a poor hot stability. Moreover, the sucrose will decompose spontaneous-ly under the acidic condition while heat doesn’t affect the decomposition of sucrose. |
</p> | </p> | ||
Revision as of 01:18, 18 October 2018


Experiment Data


Analysis
The experiment 1 illustrates the relationship between the concentration of glucose and the absorbance of the solution with DNS colorant. By using linear regression, we constructed a function with glucose concentration (μmol) as the x-axis and the ab-sorbance as the y-axis.
In the experiment 2, we measured the stability of sucrose and dextran, as well as pH stability. We let the reaction take place in the hot pot set at 100℃. We observed that dextran has a poor hot stability. Moreover, the sucrose will decompose spontaneous-ly under the acidic condition while heat doesn’t affect the decomposition of sucrose.
Analysis
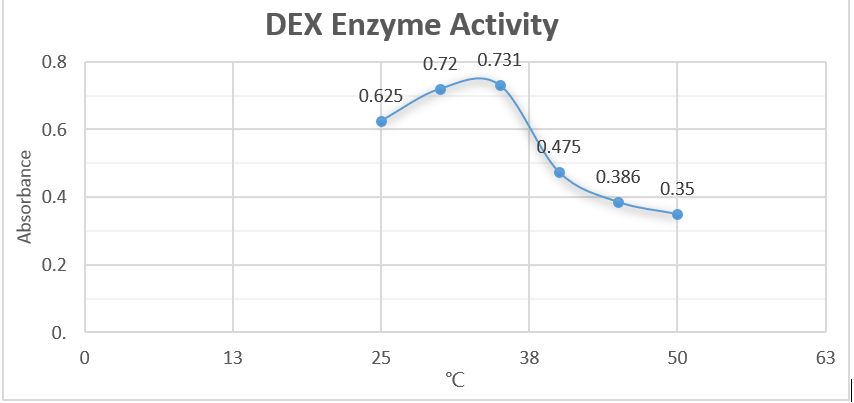
From the first half of the graph, we can clearly discover the linear relationship be-tween enzyme concentration and the absorbance. Data suggest that as the concentra-tion of enzyme becomes greater, the relative absorbance increase proportionately. This result justified our assumption. However, the latter half illustrated a negative-related relationship. We suspected that we didn’t use enough substrate to allow the decomposition reaction. Also, we assumed that excessive concentration of enzymes will negatively affected the reaction rate.

