Design
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and the third most common cause of cancer-related mortality. Despite the current use of hepatectomy and liver transplantation to improve the radical cure rates, there is still a high incidence of recurrence because of intrahepatic and extrahepatic metastases and postsurgical recurrence. The development of overt metastasis is preceded by the dissemination of tumor cells from the primary tumor to distant sites such as the blood circulation, bone marrow or lymphatic system.
Seeing that the task we hope to address had an ability to kill liver cancer cells, we decided to develop Hepashield.

The system we design is like a shield, with the combination of liver cancer-specific promoters and miRNAs, to protect human body away from liver cancer.
The sensitive system we made includes three genetic circuits:
·GAL4-VP16, an potent transcriptional activator[1], is under the control of liver cancer specific promoter.
·HSV-thymidine kinase (HSV-TK) expression is driven by Gal4-VP16 infusion protein binding to nine tandem UAS elements[2] in the promoter.
·GAL80 is a GAL4 inhibitor, whose expression is controlled under a CMV promoter as well as a cluster of liver cancer cells-specific miRNAs miRNA93/ miRNA-362-5p/ miRNA-221, binding sites at the 3’-end.
The point we have to emphasize is that the novel genetic double-switch[3] we designed makes Hepashield become much more specifically:
Switch 1: Liver cancer specific promoter AFP, hTERT and ZEB1-AS1 can motivate the expression of GAL4-VP16 infusion protein.
Switch 2: MicroRNA binding domain can control the activation of GAL80.
·In normal cells, GAL4-VP16 can not express by the inhibition of Gal80 and inactive specific promoter.

·Nevertheless, if there were no GAL80, some specific promoters may have the ability to trigger other cells that are not liver cancer cells, leading to a little bit TK expression. Therefore, the GAL80 part can dramatically weaken the activation of GAL4 protein, helping ensure the highly dormancy of HSV-TK and significantly specificity of this system.

·In liver cancer cells, some overexpressive microRNA combine with the 3’ UTR miRNA binding domain to make GAL-80 inactive.

·At the same time, there exists some specific promoters that can activate the transcription and expression of GAL4.

·Once a small dose of GAL4 have activated by their specific promoter in liver cancer cells, they can be the trigger of TK expression, achieving the tumor killing effect as gene therapy.
Only both of switch open can trigger the large quantity of HSV-TK expression. Then GCV administration is meant to eliminate liver cancer cells.
To obtain our system of well characterized mammalian digital and analog promoters, we divided our research into two technical aims:
· modify promoters to control the expression of GAL4-VP16 fusion protein (AFP, hTERT and ZEB1-AS1) and the expression of GAL80(CMV)
·integration of our parts into genetic circuit contexts
Some specific promoter can enhance the expression of Gal4-VP16 infusion. Therefore we continued to seek novel solutions with higher efficiency and convenience. Through our trail, They can be regulated with two vectors — pGL4.22 and pGL4.35.
pGL4.22 offers multiple cloning region as well encodes the luciferase reporter gene. We put CMV, AFP, TERT and ZEB1 in multiple cloning region, meanwhile make GAL4-VP16 replaces the luciferase gene.
For example, pGL4.22-AFP add promoter AFP; pGL4.22-AFP-GAL4-VP16 add AFP, then luciferase and hPEST are replaced by GAL4-VP16


pGL4.35 contains 9 repeats of GAL4 UAS (Upstream Activator Sequence), and this sequence drives transcription of the luciferase reporter gene in response to binding of a fusion protein containing the GAL4 Binding domain(BD) when activated by a ligand. Luciferase is replaced by CD-TK, also it can be replaced by PD-1[4] antibody gene.

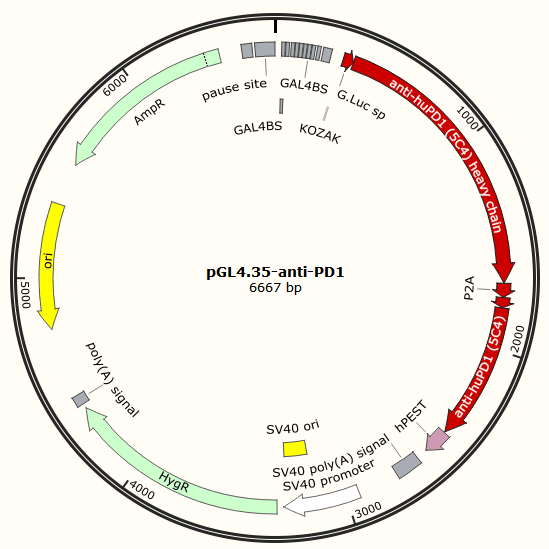
Mechanistically, HSV-TK can phosphorylate the nontoxic ganciclovir(GCV)[5][6], a cell-killing drug, and their resulting triphosphate form incorporates into DNA via the action of DNA polymerase, leading to chain termination and cell death[7]. Keytruda and Opdivo are PD-1 / PD-L1 immunotherapy drugs by Immune checkpoint therapy.
The expression of TK or PD-1 antibody gene is evaluated by MTT method.
In our work, the reporter gene we use is luciferase. The benefit of using this in luciferase assay system[8] is that they are easily assayable. Luciferase gene can show the effect and expression of the Gal4-VP16 system and HSV-TK under different promoter.
The assaying technique used throughout our research was tube luminometer (Sirius Tube Luminometer from BERTHOLD).
[1] Sadowski I, Ma J, Triezenberg S, et al. GAL4-VP16 is an unusually potent transcriptional activator[J]. Nature, 1988, 335(6190): 563.
[2] Busson D, Pret A M. GAL4/UAS targeted gene expression for studying Drosophila Hedgehog signaling[M]//Hedgehog Signaling Protocols. Humana Press, 2007: 161-201.
[3] Wieland M, Fussenegger M. Engineering molecular circuits using synthetic biology in mammalian cells[J]. Annual review of chemical and biomolecular engineering, 2012, 3: 209-234.
[4] Nissim L, Wu M R, Pery E, et al. Synthetic RNA-based immunomodulatory gene circuits for cancer immunotherapy[J]. Cell, 2017, 171(5): 1138-1150. e15.
[5] Willmon C L, Krabbenhoft E, Black M E. A guanylate kinase/HSV-1 thymidine kinase fusion protein enhances prodrug-mediated cell killing[J]. Gene therapy, 2006, 13(17): 1309.
[6] Greco R, Oliveira G, Stanghellini M T L, et al. Improving the safety of cell therapy with the TK-suicide gene[J]. Frontiers in pharmacology, 2015, 6: 95.
[7] Jones B S, Lamb L S, Goldman F, et al. Improving the safety of cell therapy products by suicide gene transfer[J]. Frontiers in pharmacology, 2014, 5: 254.
[8] Barriscale K A, O’Sullivan S A, McCarthy T V. A single secreted luciferase-based gene reporter assay[J]. Analytical biochemistry, 2014, 453: 44-49.


