Interlab
Inerlab has come to the fifth session as a yardstick for measuring whether the standards of various laboratories around the world meet the requirements. Through standard curve measurement, 96-well plate readings and colony calculations, teams are able to ensure the measurement capabilities of each laboratory. Interlab not only provides a basis for comparison between different laboratories, but also provides a basis for accurate research in synthetic biology.
Positive Control (BBa_I20270): well 2B
Negative Control (BBa_R0040): well 2D
Test Device 1 (BBa_J364000): well 2F
Test Device 2 (BBa_J364001): well 2H
Test Device 3 (BBa_J364002): well 2J
Test Device 4 (BBa_J364007): well 2L
Test Device 5 (BBa_J364008): well 2N
Test Device 6 (BBa_J364009): well 2P
Escherichia coli strain DH5α
1ml LUDOX
ddH2O
10ml 1X PBS
LB (Luria Bertani) media
Chloramphenicol (stock concentration 25 mg/mL dissolved in EtOH)
Pipettes
96-well plate
50 ml Falcon tube (or equivalent, preferably amber or covered in foil to block light)
1.5-ml Eppendorf tubes for sample storage, ice bucket with ice
Micropipettes and tips
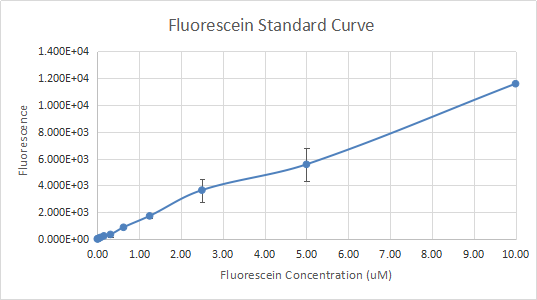
There are 3 different parts of Interlab, including calibration, cell measurement and colony forming units. Calibrations are made up of OD6 00 Reference points, Particle Standard Curve, and Fluorescence standard curve, using LUDOX CL-X, Silica beads – Microsphere, Fluorescein provided in kit to generate a standardized curve. Cell measurement has 8 plasmids (Positive Control, Negative Control, Test Device 1, Test Device 2, Test Device 3, Test Device 4, Test Device 5, and Test Device 6) from Kit Plate 7 were transformed into E. coli DH5-alpha cells. After transformation and cultivation, the cells were used for experiments through the protocol. The third part is a procedure that aims to calibrate OD600 to colony forming unit (CFU) counts, which are directly relatable to the cell concentration of the culture.