Project
Design
Plasmid Design
This proof-of-principle project required the design and construction of two expression plasmids:
- An expression plasmid for an odorant receptor protein
- A plasmid containing a synthetic operon
Design of odorant receptor protein expression plasmid
The human OR1D2 receptor (hOR1D2) protein will be used because it is encoded by a single open reading frame that is compatible with iGEM parts submission restrictions. The hOR1D2 open reading frame will be amplified from a template plasmid for mammalian cell expression (which was obtained OriGene). Production of the OR1D2 PCR product will be verified by PCR, and then digested with EcoRI and HindIII restriction enzymes.
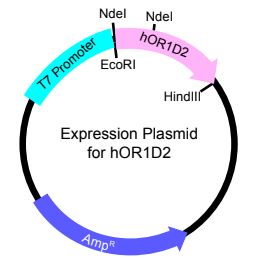
Figure 1
The pET-21A plasmid will be used to express the hOR1D2 protein because its expression in this plasmid will be controlled by the T7 promoter (Figure 1), which requires induction with IPTG. This system will allow us to have temporal control of hOR1D2 expression in our cultures. The pET-21A will be obtained from Dr. Cave and digested with EcoRI and HindIII. The digested hOR1D2 and pET21A insert and plasmid, respectively, will be ligated to create the desired expression vector. DH5alpha cells will be transformed with the ligation reactions, and plasmid isolated from the colonies will be screened by restriction digest reactions to identify plasmids that have the insert.
Design of synthetic operon plasmid
The synthetic operon in this proof-of-principle project will be constructed with a pUC57 plasmid backbone. To drive expression of the operon genes, the AracC-Pbad promoter available in the iGEM parts database (part BBa_K808000) will be used to drive operon gene expression (Figure 2), which will require the addition of exogenous arabinose. Similar to the use of the T7 promoter, the AracC-Pbad promoter will allow us to have temporal control over the expression of the operon genes in our cultures. The synthetic operon will drive the expression of the human Tau and bacterial icaB genes. These proteins will be fused with His and Strep tag amino acids, respectively, which will facilitate detection of their expression in our cultures. The plasmid containing this synthetic operon will be constructed by GeneWiz. Once generated, DH5alpha cells will be transformed with this plasmid to generate a bacterial stock for further amplification of this plasmid.
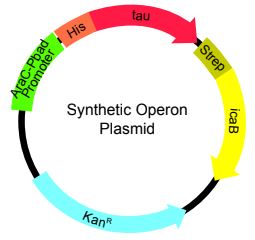
Figure 2
Expression of odorant and synthetic operon proteins
The hOR1D2-pET21A and synthetic operon plasmids will be used to co-transform BL21(DE3) cells. To establish co-expression, transformed BL21(DE3) cells will be grown in minimal media using glycerol as a carbon source to an OD600=0.6, and then induced with IPTG and arabinose. As controls, additional cultures will be grown and receive either IPTG only, arabinose only, or nothing. All cultures will be grown for an additional 3 hours before being harvested for RNA. Quantitative RT-PCR will be performed to measure hOR1D2 and synthetic operon gene expression levels in the different cultures.


