| Line 41: | Line 41: | ||
<h1>Colony Forming Units per 0.1 OD<sub>600</sub><i>E. coli</i> cultures</h1> | <h1>Colony Forming Units per 0.1 OD<sub>600</sub><i>E. coli</i> cultures</h1> | ||
<p>For the CFU protocol, wecalculated colonies for our two Positive Control (BBa_I20270) cultures and our twoNegative Control (BBa_R0040) cultures.Our overnight cultureswere diluted to yield a 0.1 OD<sub>600</sub> using calculations described in iGEM 2018 InterLab Study Protocol.After a serial dilution for each overnight culture, we platedthe 8*10<sup>-3</sup>, 8*10<sup>-4</sup>, and 8*10<sup>-5</sup> dilutions on LB agar with triplication.With the assumption that one bacterial cell leads to one colony, we enumerated the number of colonies on each plate and multiplied the colony count by the final dilution factor to assess a CFU/mL value for a starting sample of OD<sub>600</sub>.</p> | <p>For the CFU protocol, wecalculated colonies for our two Positive Control (BBa_I20270) cultures and our twoNegative Control (BBa_R0040) cultures.Our overnight cultureswere diluted to yield a 0.1 OD<sub>600</sub> using calculations described in iGEM 2018 InterLab Study Protocol.After a serial dilution for each overnight culture, we platedthe 8*10<sup>-3</sup>, 8*10<sup>-4</sup>, and 8*10<sup>-5</sup> dilutions on LB agar with triplication.With the assumption that one bacterial cell leads to one colony, we enumerated the number of colonies on each plate and multiplied the colony count by the final dilution factor to assess a CFU/mL value for a starting sample of OD<sub>600</sub>.</p> | ||
| − | |||
| − | |||
| − | |||
<img class="myimg" src="https://static.igem.org/mediawiki/2018/e/e2/T--Biomarvel--inter8.png"> | <img class="myimg" src="https://static.igem.org/mediawiki/2018/e/e2/T--Biomarvel--inter8.png"> | ||
<h4 style="text-align: center;">Fig 8. Plated DH5α cells of serial dilutions for starting samples</h4> | <h4 style="text-align: center;">Fig 8. Plated DH5α cells of serial dilutions for starting samples</h4> | ||
Latest revision as of 11:05, 30 September 2018

The InterLab study is organized by a measurement committee of iGEM to establish better measurement techniques in the synthetic biology field.We submit our measurement results that cope with the absorbance and fluorescence related withE. coli transformed with a variety oftest devices. We checked 8 plasmids, containing 2 controls and 6 test devices, and measurements were made in a plate reader with a 96 well microplate. We measured the absorbance and fluorescence of our samples using a Perkin Elmer 1420 multilabel counter.
Calibration 1: OD 600 Reference point - LUDOX Protocol
We used LUDOX CL-X as a single point reference to obtain a conversion factor to transform absorbance (Abs 600) data fromplate reader into acomparable OD 600 measurement as would be obtained in a spectrophotometer.

Fig 1. Measurement of Abs 600 and OD 600 for LUDOX CL-Xand water
Calibration 2: Particle Standard Curve - Microsphere Protocol
We prepared a dilution series of silica microspheres and measure the Abs 600 in a plate reader. These microspheres are similar to cells in physical size and optical characteristics. This measurement was used to obtain a standardcurve of particle concentration, enabling to convert Abs 600 measurements to an estimatednumber of cells.
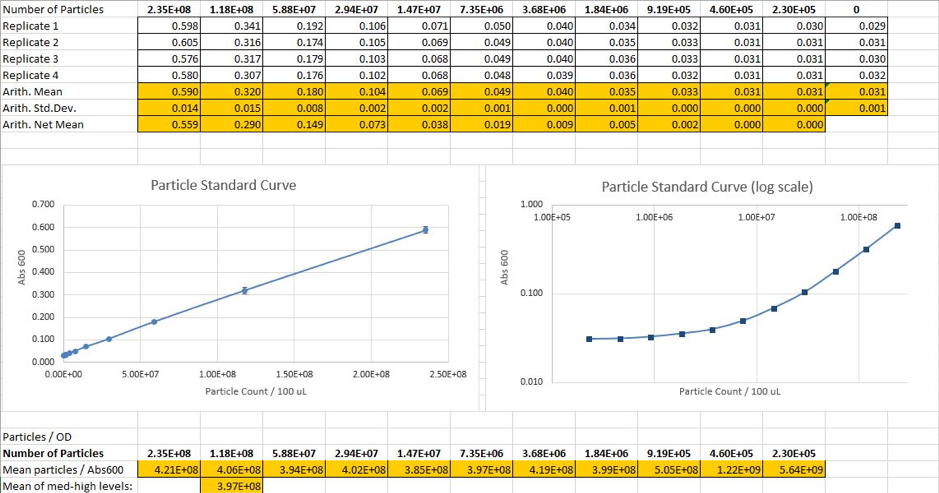
Fig2. Calculated particle counts from measured Abs600and generation of a particle standard curve
Calibration 3: Fluorescence standard curve - Fluorescein Protocol
We prepared a dilution series of fluorescein in four replicates and measure the fluorescence in a96 well plate in aplate reader. Measuring these in the plate readerenabled us togenerate astandard curve of fluorescence for fluorescein concentration.

Fig 3.Correspondence between fluorescence and fluorescein concentration
Cell measurement
We had two plates to read at the end of procedure and one plate for each time point: 0 and 6 hours. On each plate,we readboth fluorescence and absorbance.We used the generated particle standard and fluorescent curves to determine fluorescence per OD and fluorescence per particle values.

Fig 4. Measurement of raw Abs600 and OD600
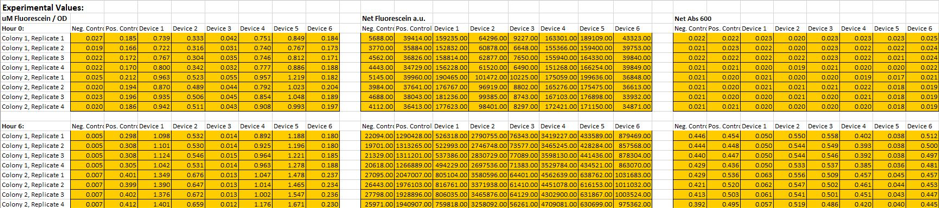
Fig 5. Measurement of fluorescence per OD

Fig 6. Measurement of fluorescence per particle

Fig 7. The number of colonies and CFU/mL value in a starting sample of OD600 = 0.1
Colony Forming Units per 0.1 OD600E. coli cultures
For the CFU protocol, wecalculated colonies for our two Positive Control (BBa_I20270) cultures and our twoNegative Control (BBa_R0040) cultures.Our overnight cultureswere diluted to yield a 0.1 OD600 using calculations described in iGEM 2018 InterLab Study Protocol.After a serial dilution for each overnight culture, we platedthe 8*10-3, 8*10-4, and 8*10-5 dilutions on LB agar with triplication.With the assumption that one bacterial cell leads to one colony, we enumerated the number of colonies on each plate and multiplied the colony count by the final dilution factor to assess a CFU/mL value for a starting sample of OD600.








