HANSONGTSENG (Talk | contribs) |
HANSONGTSENG (Talk | contribs) |
||
| Line 44: | Line 44: | ||
<p>For the FRET pair, we have chosen CyPet and YPet. The FRET pair is a cyan fluorescent protein and yellow fluorescent protein optimized for FRET reactions. After deciding the protein pair to use, each of the protein will be fused with CyPet or YPet, a fluorescent protein pair developed with enhanced FRET efficiency [3]. The peak excitation and emission wavelengths of CyPet and YPet are 414 and 475nm, 515 and 530nm, respectively [4]. Something to notice is that in order to minimize the destruction on protein structure during purification, the His tag is added to the fluorescent protein instead of the binding protein. This leads us to the design of two kinds of plasmids shown below: | <p>For the FRET pair, we have chosen CyPet and YPet. The FRET pair is a cyan fluorescent protein and yellow fluorescent protein optimized for FRET reactions. After deciding the protein pair to use, each of the protein will be fused with CyPet or YPet, a fluorescent protein pair developed with enhanced FRET efficiency [3]. The peak excitation and emission wavelengths of CyPet and YPet are 414 and 475nm, 515 and 530nm, respectively [4]. Something to notice is that in order to minimize the destruction on protein structure during purification, the His tag is added to the fluorescent protein instead of the binding protein. This leads us to the design of two kinds of plasmids shown below: | ||
</p> | </p> | ||
| + | <img src="https://static.igem.org/mediawiki/2018/d/d9/T--NYMU-Taipei--design-CyPetYPetFRET.png"> | ||
| + | |||
<h2>How do we determine the amount?</h2> | <h2>How do we determine the amount?</h2> | ||
Revision as of 03:28, 17 October 2018

Cell system
FRET system
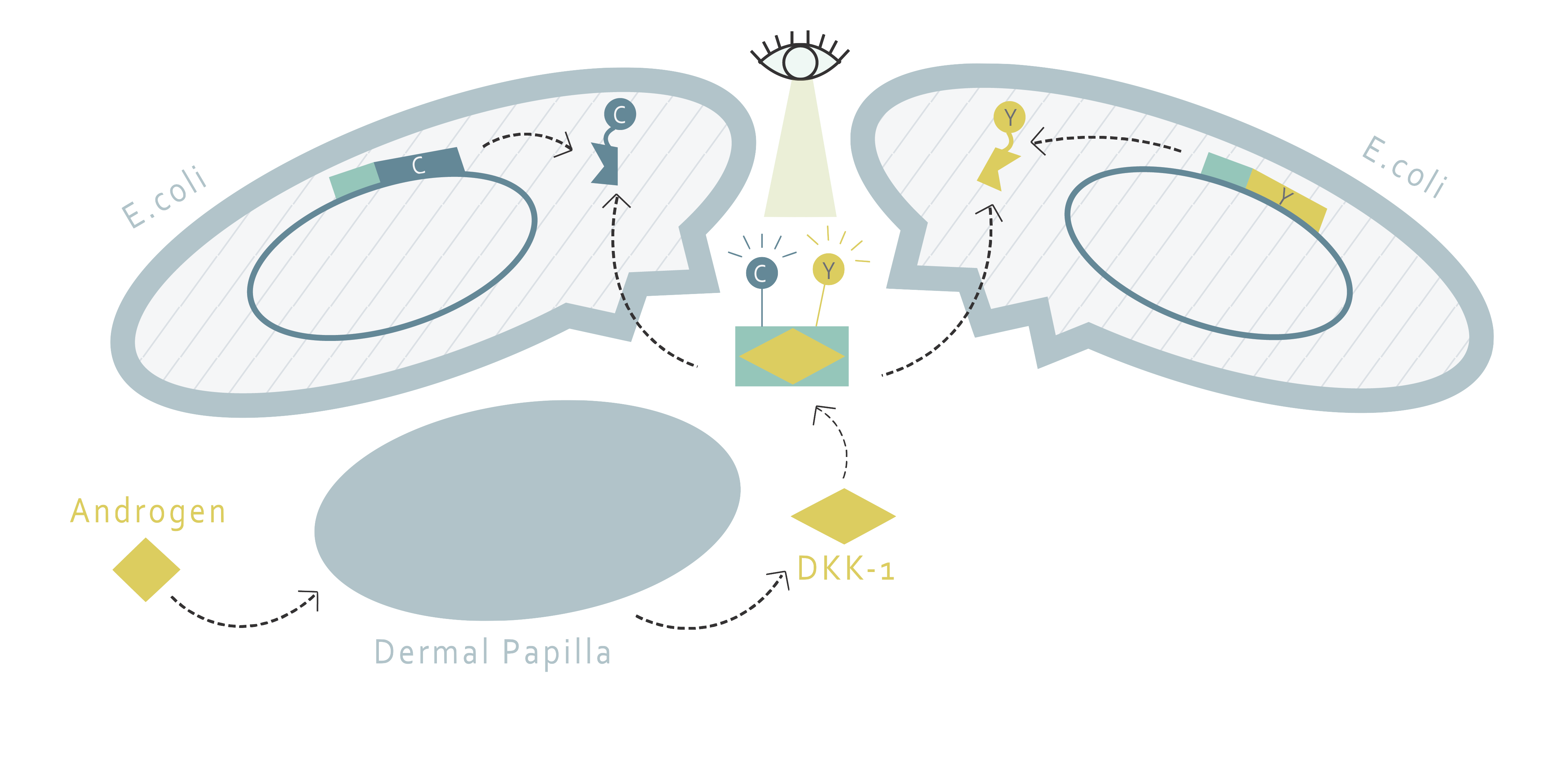
How do we establish a drug screening system without creating a new cell line ?
This part of our project aims to determine the amount of DKK1 in cell supernatants through fluorescence resonance energy transfer (FRET). FRET requires two molecules: a donor and an acceptor. If the emission wavelength of the donor molecule and the absorption wavelength of the acceptor molecule overlap each other, the excitation of the donor molecule will result in the fluorescence emission of the acceptor molecule when the two molecules are nearby. Therefore, the FRET system allows us to determine whether or not two molecules are close, or most likely, interacting with each other.

Using this method, we would only need to find a FRET pair and two proteins that bind to DKK1 and fusion them in the form of one FRET protein to one binding protein. This fusion pair can be produced by E.coli and purified through protein purification. Afterwards, with proper buffer, one would only have to add the fusion proteins into the supernatant of cells and observe the fluorescence pattern to determine the amount of DKK1.
How can we bind the FRET pairs to DKK1?
Our design requires two proteins to interact with DKK1 simultaneously by binding to different binding sites on DKK1. There are several potential candidate proteins that we can choose from. G5 and H7 are nanobodies that have been proven to bind to DKK1[1]. Another option is the low-density lipoprotein receptor-related protein 6 (LRP6). LRP6 is a protein involved in the Wnt pathway, and is regulated by DKK1 binding. It contains four subdomains, including four EGF-like domains (E1-4). However, the size of the whole LRP6 protein is too big, and is not suitable for FRET purposes. According to literature, dividing LRP6 into LRP6-E1E2 and LRP6-E3E4 does not interfere with their abilities to bind to DKK1[2]. Moreover, LRP6-E1 and LRP6-E3 propellers are suspected to play a crucial role in DKK1 binding. The best protein pair for DKK1 binding is determined by experiment.
What FRET pairs should we use?
For the FRET pair, we have chosen CyPet and YPet. The FRET pair is a cyan fluorescent protein and yellow fluorescent protein optimized for FRET reactions. After deciding the protein pair to use, each of the protein will be fused with CyPet or YPet, a fluorescent protein pair developed with enhanced FRET efficiency [3]. The peak excitation and emission wavelengths of CyPet and YPet are 414 and 475nm, 515 and 530nm, respectively [4]. Something to notice is that in order to minimize the destruction on protein structure during purification, the His tag is added to the fluorescent protein instead of the binding protein. This leads us to the design of two kinds of plasmids shown below:

How do we determine the amount?
When DKK1 is present, we hypothesize that the selected proteins will both bind to DKK1, bringing CyPet and YPet close enough for FRET to happen. By detecting the strength of fluorescence emission, we can thereby determine the amount of DKK1 proteins existing in the supernatant.

