| (5 intermediate revisions by one other user not shown) | |||
| Line 77: | Line 77: | ||
<p>Another group of patients in need of new treatment options are people with burn wounds. In the Netherlands, the number of patients hospitalized with severe burns is approximately 650 annually, but many more suffer from smaller burns. During our visit to the largest burn wound center in the Netherlands, we learned that sterile burn wounds do not exist. Treatment is thus about maintaining bacterial levels within an acceptable range. Burn wounds are in that respect comparable in terms of risk with the chronic wounds discussed above. Both wound types are most commonly infected with <i>Staphylococcus aureus</i>. For burn wounds, <i>Pseudomonas aeruginosa</i> comes second, whereas <i>Streptococci</i> and <i>Pseudomonas</i> are both commonly found on diabetic wounds according to the medical professionals we talked to.</p> | <p>Another group of patients in need of new treatment options are people with burn wounds. In the Netherlands, the number of patients hospitalized with severe burns is approximately 650 annually, but many more suffer from smaller burns. During our visit to the largest burn wound center in the Netherlands, we learned that sterile burn wounds do not exist. Treatment is thus about maintaining bacterial levels within an acceptable range. Burn wounds are in that respect comparable in terms of risk with the chronic wounds discussed above. Both wound types are most commonly infected with <i>Staphylococcus aureus</i>. For burn wounds, <i>Pseudomonas aeruginosa</i> comes second, whereas <i>Streptococci</i> and <i>Pseudomonas</i> are both commonly found on diabetic wounds according to the medical professionals we talked to.</p> | ||
<h3><i>Staphylococcus aureus</i></h3> | <h3><i>Staphylococcus aureus</i></h3> | ||
| − | <p><i>Staphylococcus</i> was unanimously mentioned by our stakeholders as the most problematic bacterial strain to remove from wounds. <i>S. aureus</i> is even found on over 90% of the chronic venous ulcers, which is the most common type of chronic ulcers (8). The exact numbers differ per country. <i>S. aureus</i> is | + | <p><i>Staphylococcus</i> was unanimously mentioned by our stakeholders as the most problematic bacterial strain to remove from wounds. <i>S. aureus</i> is even found on over 90% of the chronic venous ulcers, which is the most common type of chronic ulcers (8). The exact numbers differ per country. <i>S. aureus</i> is becoming increasingly resistant to antibiotics (5). The most well-known antibiotic-resistant type of <i>S. aureus</i> is methicillin-resistant <i>S. aureus</i> (MRSA), which has been emerging since the 1960s. In the United States, MRSA causes approximately 11,000 deaths annually, which is more than HIV/AIDS, emphysema, Parkinson’s disease, and homicide combined (9,10). The Netherlands have been more successful at preventing MRSA dissemination, but MRSA still occurs (11). Furthermore, other antibiotic-resistant strains of <i>S. aureus</i> are encountered, such as vancomycin-resistant <i>S. aureus</i> (9). Multi-resistant strains are also becoming a common phenomenon (12). Dr. Dokter from the Maasstad Hospital additionally emphasized that he increasingly encounters toxin-producing and resistant strains of <i>S. aureus</i>, which are more aggressive than ‘regular’ strains.</p> |
<h3>Current treatments</h3> | <h3>Current treatments</h3> | ||
<div class="figureWrapper figureRight"> | <div class="figureWrapper figureRight"> | ||
| Line 86: | Line 86: | ||
</a> | </a> | ||
<div class="figureCaption"> | <div class="figureCaption"> | ||
| − | <p>Figure 1. A skin wound with | + | <p>Figure 1. A skin wound with different treatment approaches: antibiotics delivered locally from a cream or systemically from the blood. |
</p> | </p> | ||
</div> | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
| − | <p>Unfortunately, the treatment of diabetic wounds as well as burn wounds is hampered by the mentioned surge of antibiotic-resistant bacteria. Additional to the rise of resistance is the problem that pharmaceutical companies have abandoned antibiotics research over the past decades. | + | <p>Unfortunately, the treatment of diabetic wounds as well as burn wounds is hampered by the mentioned surge of antibiotic-resistant bacteria. Additional to the rise of resistance is the problem that pharmaceutical companies have abandoned antibiotics research over the past decades. From an economical perspective, it is not interesting for them (13). As the discovery of new antibiotics has drastically slowed down since the 1980s, new types of antibiotics cannot be expected to overcome this problem (10).</p> |
<p>During visits to several wound clinics and medical specialists (see <a href="https://2018.igem.org/Team:TU-Eindhoven/Human_Practices">Integrated Practices</a></li>), we learned about other treatment options. In addition to antibiotics, doctors use range of antiseptic bandages and creams. However, most of them are ineffective according to Dr. Peters, a specialist in diabetic wounds. Mechanical cleaning is still an important part of (burn) wound treatments, but it is painful for the patient and labor-intensive. Furthermore, doctors often resort to systemic antibiotics, especially before the type of infection has been diagnosed. Dr. Dokter complained that non-specific antibiotics are often ineffective. In addition, their use contributes to the development of antibiotic resistance. Moreover, it negatively affects patients’ gut microbiome, which in the case of diabetic patients is already frequently disturbed. Other side-effects such as Karel’s kidney and liver problems are also commonly reported (14). </p> | <p>During visits to several wound clinics and medical specialists (see <a href="https://2018.igem.org/Team:TU-Eindhoven/Human_Practices">Integrated Practices</a></li>), we learned about other treatment options. In addition to antibiotics, doctors use range of antiseptic bandages and creams. However, most of them are ineffective according to Dr. Peters, a specialist in diabetic wounds. Mechanical cleaning is still an important part of (burn) wound treatments, but it is painful for the patient and labor-intensive. Furthermore, doctors often resort to systemic antibiotics, especially before the type of infection has been diagnosed. Dr. Dokter complained that non-specific antibiotics are often ineffective. In addition, their use contributes to the development of antibiotic resistance. Moreover, it negatively affects patients’ gut microbiome, which in the case of diabetic patients is already frequently disturbed. Other side-effects such as Karel’s kidney and liver problems are also commonly reported (14). </p> | ||
</section> | </section> | ||
| Line 261: | Line 261: | ||
<section> | <section> | ||
<h2>The future of our living material</h2> | <h2>The future of our living material</h2> | ||
| − | <p>In our iGEM project, we demonstrated our living material as a useful platform for wound healing. However, we envision our living material to be a much broader platform useful for a range of applications. Living materials have the potential to perform extraordinary functions. We invite future iGEM teams to use our platform for their own creative applications. Team <a href="https://2018.igem.org/Team:Hamburg/Collaborations">Hamburg</a></li> | + | <p>In our iGEM project, we demonstrated our living material as a useful platform for wound healing. However, we envision our living material to be a much broader platform useful for a range of applications. Living materials have the potential to perform extraordinary functions. We invite future iGEM teams to use our platform for their own creative applications. Team <a href="https://2018.igem.org/Team:Hamburg/Collaborations">Hamburg</a></li> has already investigated our hydrogel for their own project. We hope that more applications will follow!</p> |
</section> | </section> | ||
<section> | <section> | ||
<h2>Acknowledgements</h2> | <h2>Acknowledgements</h2> | ||
| − | <p>The design of the living material and the wound patch has been informed by interactions with several stakeholders. We described these interactions on the integrated human practices page . However, we would like to once again thank all of them for their valuable input. Our project would not have been the same without you!</p> | + | <p>The design of the living material and the wound patch has been informed by interactions with several stakeholders. We described these interactions on the integrated human practices page. However, we would like to once again thank all of them for their valuable input. Our project would not have been the same without you!</p> |
<p>We want to thank the Dutch National Institute for Public Health and the Environment (RIVM), the Dutch Institute for Technology Assessment (RIVM), PAMM, Maasstad Hospital Burn Wound Center, Plasmacure, Expertise Center Wound Care, chronic wound patient Karel, Dr. Edgar Peters (VUmc), Prof. Dr. Menno Prins (TU/e), Prof. Dr. Jan van Hest (TU/e), Prof. Dr. Patricia Dankers (TU/e), Dr. Lily Frank (TU/e), Dr. Sandra Hofmann (TU/e), Lesley Bouwer, M.D., iGEM Düsseldorf 2018 and finally the scientists and professionals we met at the Netherlands Biotechnology Congress 2018.</p> | <p>We want to thank the Dutch National Institute for Public Health and the Environment (RIVM), the Dutch Institute for Technology Assessment (RIVM), PAMM, Maasstad Hospital Burn Wound Center, Plasmacure, Expertise Center Wound Care, chronic wound patient Karel, Dr. Edgar Peters (VUmc), Prof. Dr. Menno Prins (TU/e), Prof. Dr. Jan van Hest (TU/e), Prof. Dr. Patricia Dankers (TU/e), Dr. Lily Frank (TU/e), Dr. Sandra Hofmann (TU/e), Lesley Bouwer, M.D., iGEM Düsseldorf 2018 and finally the scientists and professionals we met at the Netherlands Biotechnology Congress 2018.</p> | ||
</section> | </section> | ||
Latest revision as of 18:30, 7 December 2018
Product Design
The best that most of us can hope to achieve in physics is simply to misunderstand at a deeper level.
The problem of wound infections
Wound infections are an attractive target for living materials. During a visit to Expertisecentrum Wondzorg, a center specialized in chronic wounds, we met patient Karel. He had been suffering from chronic wounds for nine months. Because of diabetes and an impaired vascular system, his wounds heal poorly. Karel was so kind to share his story with us.
Due to wounds on my feet I can barely walk. After the removal of dead tissue, the wounds got deeper and the pain worse. Generally, I am always afraid that the mechanical cleaning of my wounds makes them expand or deeper. One of the wounds on my feet got infected and took six weeks of systemic antibiotics to get rid of. This had a great impact on my well-being. In particular, the impact of the antibiotics on my liver and kidney was heavy.
Scope of the problem
There are more patients like Karel. Only in the Netherlands (population size: 17 million), an estimated 500.000 people suffer from complex wounds (4). Globally, 1-2% of the population is affected by chronic leg ulcers (5). Practically all chronic wounds are infected with bacteria. Thirty percent of the wounds will not close, putting patients at risk of amputation. One group of people particularly suffering from wound infections are diabetic patients. Due to poor blood flow to extremities, patients easily develop wounds which subsequently heal poorly and get infected. Other patients at risk of developing chronic wounds are (elderly) people suffering from vascular diseases. Apart from patient suffering, costs to society are large, with an annual estimate of 3.2 billion euros of medical expenditure (6). As the group of affected patients will increase due to aging population, costs are expected to rise (7).
Another group of patients in need of new treatment options are people with burn wounds. In the Netherlands, the number of patients hospitalized with severe burns is approximately 650 annually, but many more suffer from smaller burns. During our visit to the largest burn wound center in the Netherlands, we learned that sterile burn wounds do not exist. Treatment is thus about maintaining bacterial levels within an acceptable range. Burn wounds are in that respect comparable in terms of risk with the chronic wounds discussed above. Both wound types are most commonly infected with Staphylococcus aureus. For burn wounds, Pseudomonas aeruginosa comes second, whereas Streptococci and Pseudomonas are both commonly found on diabetic wounds according to the medical professionals we talked to.
Staphylococcus aureus
Staphylococcus was unanimously mentioned by our stakeholders as the most problematic bacterial strain to remove from wounds. S. aureus is even found on over 90% of the chronic venous ulcers, which is the most common type of chronic ulcers (8). The exact numbers differ per country. S. aureus is becoming increasingly resistant to antibiotics (5). The most well-known antibiotic-resistant type of S. aureus is methicillin-resistant S. aureus (MRSA), which has been emerging since the 1960s. In the United States, MRSA causes approximately 11,000 deaths annually, which is more than HIV/AIDS, emphysema, Parkinson’s disease, and homicide combined (9,10). The Netherlands have been more successful at preventing MRSA dissemination, but MRSA still occurs (11). Furthermore, other antibiotic-resistant strains of S. aureus are encountered, such as vancomycin-resistant S. aureus (9). Multi-resistant strains are also becoming a common phenomenon (12). Dr. Dokter from the Maasstad Hospital additionally emphasized that he increasingly encounters toxin-producing and resistant strains of S. aureus, which are more aggressive than ‘regular’ strains.
Current treatments
Unfortunately, the treatment of diabetic wounds as well as burn wounds is hampered by the mentioned surge of antibiotic-resistant bacteria. Additional to the rise of resistance is the problem that pharmaceutical companies have abandoned antibiotics research over the past decades. From an economical perspective, it is not interesting for them (13). As the discovery of new antibiotics has drastically slowed down since the 1980s, new types of antibiotics cannot be expected to overcome this problem (10).
During visits to several wound clinics and medical specialists (see Integrated Practices), we learned about other treatment options. In addition to antibiotics, doctors use range of antiseptic bandages and creams. However, most of them are ineffective according to Dr. Peters, a specialist in diabetic wounds. Mechanical cleaning is still an important part of (burn) wound treatments, but it is painful for the patient and labor-intensive. Furthermore, doctors often resort to systemic antibiotics, especially before the type of infection has been diagnosed. Dr. Dokter complained that non-specific antibiotics are often ineffective. In addition, their use contributes to the development of antibiotic resistance. Moreover, it negatively affects patients’ gut microbiome, which in the case of diabetic patients is already frequently disturbed. Other side-effects such as Karel’s kidney and liver problems are also commonly reported (14).
Requirements
Considering the problem of antibiotic resistance and the ineffectiveness of alternative treatments, we need a treatment which is specific, local and does not use antibiotics.
Specificity
The specificity requirement leads us to bacteriocins. Bacteriocins are antimicrobial peptides naturally produced by a large variety of bacteria. They normally target closely related strains. The bacteriocin comes with an immunity mechanism which protects the producing strain from the bacteriocin’s activity (15). Specific bacteriocins are already used as preservatives in the food industry, others are tested for use in pharmaceutical applications. Thanks to the rise in (multiple) antibiotic-resistant pathogens, bacteriocins have gained renewed interested over the past two decades (15). Interestingly, resistance against bacteriocins is not common, especially in comparison to conventional antibiotics. The resistance frequencies however differ greatly per bacteriocin (16).
As we had recognized S. aureus as the most common cause of wound infections, we searched for a suitable bacteriocin targeting it. A constraint in the search was that the selected bacteriocin cannot be not toxic to E. coli. Lysostaphin is a bacteriolytic enzyme which cleaves the cell wall of Staphylococci (18). Previously, it has been demonstrated that lysostaphin treatment eradicated regular and antibiotic-resistant S. aureus in bone fractures (18). It had already been shown that lysostaphin can be safely produced by E. coli (19). Importantly, resistance against lysostaphin is not commonly detected and there are indications that it comes with reduced fitness and increased susceptibility to other antibiotics (20). We thus selected lysostaphin as our main bacteriocin for this project. For more information on lysostaphin’s functioning and the mechanism by which it is secreted, please have a look at our Design page.
Local release
To release lysostaphin locally to the wound, several approaches are possible. In 2016, teams from SDU Denmark as well as from Stockholm designed wound dressings from recombinant spider silk functionalized with antimicrobial peptides. However, the teams described the silk production process as “challenging”. Alternatively, one could pack lysostaphin in a hydrogel. Hydrogels are polymers which swell in contact with water. Thanks to their biocompatibility, similarity to the extracellular matrix and flexibility in chemical and physical properties, they are popular drug delivery vehicles. Their chemical properties can be adapted to bind the drug of interest (21). However, a disadvantage of loading a hydrogel with functional proteins is that the gel will eventually get depleted. Furthermore, the limited half-life of proteins such as lysostaphin will affect their effectivity (18).
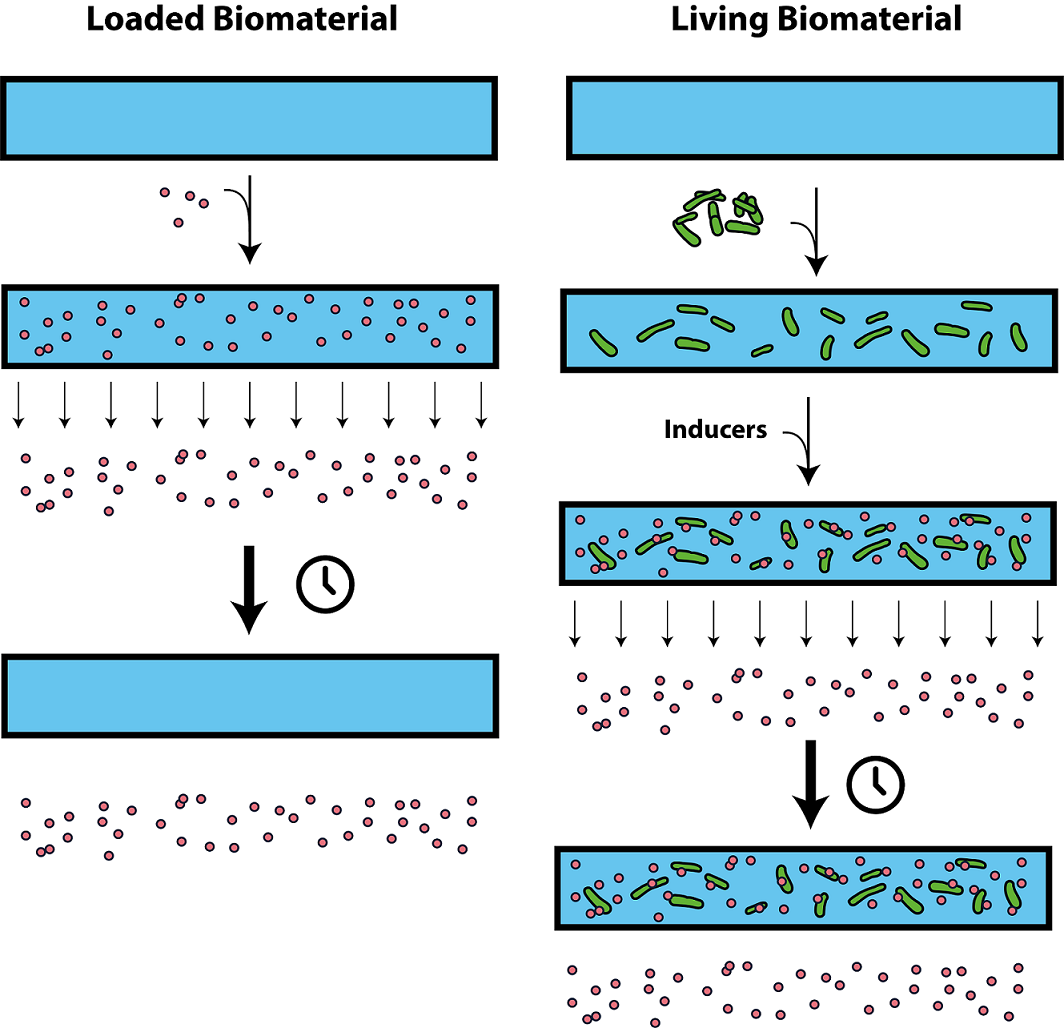
An interesting novel approach for sustained release would be the use of a living material. Living materials are a combination of a biocompatible material and living organisms. Both would be specifically chosen and engineered to perform a task as a single device. Such devices could range from drug delivery vehicles to toxicity sensors. An important advantage of the combination of living cells with materials, is the ability of cells to be programmed to compute and to respond to complex external stimuli (1). In our case, the living material can release lysostaphin in response to environmental stimuli such as induction by medical professionals or based on quorum sensing (see Outlook). In contrast to regular biomaterials used as drug delivery vehicles, living materials are suitable for sustained release (figure 3).
Several groups have endeavored the construction of living materials. Attempted strategies include the 3D printing of hydrogels with living (bacterial) cells, the incorporation of yeast or bacteria between a polymer support and a nanoporous top layer and the integration of bacteria in hydrogel-elastomer hybrids (3,22–24). Those attempts successfully resulted in the construction of responsive, wearable patches such as penicillin-producing layers. Yet, it remains challenging to create hybrid living materials which maintain sustained cell viability, allow intercellular and environmental communication, and guarantee the safe entrapment of cells inside the material (1). To date, most attempts involved caging bacteria behind a nanoporous top layer, thus limiting the diffusion of nutrients, signaling molecules and secreted products. Team iGEM Groningen 2014 tried a similar approach in their design of a burn wound patch. They used a mechanical support combined with a nanoporous membrane and a top membrane to prevent escape. Their lack of bacterial proliferation in polyacrylamide (pAA) gels may be a consequence of the limited diffusion caused by such a system, considering that they did observe growth in ruptured gels. The example illustrates that viability remains a key challenge in the design of living materials, a problem apparent in 3D printed gels with tiny pores. This approach greatly limits the range of possible applications. As stated by Liu et al., “A versatile material system and a general method to design living materials (…) remain a critical need in the field” (1).
Design & Results
Design
To maintain bacterial viability while ensuring containment, we take a new approach to living material design. Instead of limiting the pore size of the hydrogel, we equip our bacteria with a special adhesion protein with affinity for a specific hydrogel to entrap them inside. Consequently, we are free to adapt the material’s pore size to the requirements for bacterial viability and functionality. The larger pore size enables the free diffusion of nutrients, signaling molecules and targeted products into and out of the gel. This strategy thus ensures the material’s safety, viability and functionality.
We thus searched for a specific combination of a biocompatible hydrogel and an adhesin with high affinity for the gel to meet the (bio)safety requirement. In addition, we needed a robust gel which provides a viable environment to the E. coli bacteria we incorporate into the material. It must be possible to sterilize the gel. Preferentially, the material should also be affordable, well-storable, sustainable and its synthesis should be straight-forward, safe and scalable. As documented on the design page , the search led us to the combination of a dextran hydrogel with a long sugar-binding adhesin derived from the Antarctic bacterium Marinomonas primoryensis. The adhesin naturally binds glucose with high affinity (25). It is thus a good match with dextran, which is a polymer of glucose.
Dextran is biocompatible and has a high porosity which ensures bacterial viability. It is also freeze-dryable and has a high diffusivity (26). Dextran has been demonstrated to be suitable for tissue-engineering, and is thus expected to be suitable for biomedical applications (27). It is also relatively affordable (see our cost calculation below) and the synthesis procedure is relatively straightforward. Furthermore, it can be easily sterilized during polymerization (28). Finally, the gel has been proven robust even under harsh conditions. Besides our own efforts, Team Hamburg tried to dissolve our gel under several chemical conditions, but had difficulties finding a successful strategy. The synthesis does however require toxic components. To minimize the safety risk for manufacturers and allow easy upscaling, we researched alternative procedures as part of our safe-by-design principle. For more information, please check our Safe-by-Design .
To secrete lysostaphin, we made E. coli produce lysostaphin fused to a secretion tag compatible with the secretion pathway used for the adhesin. This allows for the efficient secretion of both lysostaphin and the adhesin while minimizing the burden on the bacteria. The different components of our system (adhesin, secretion system, lysostaphin) were designed to be individually inducible for optimal control over all components.
Results
Our microscopy results confirmed the high porosity of the dextran hydrogels. The structure seemed suitable for bacterial viability, as viability experiments indicated that large numbers of E. coli bacteria survive in the hydrogels for at least a week. Future experiments will need to provide more accurate results on the survival rate. Furthermore, it would be interesting to test survival over longer periods of time. Nevertheless, the observed survival is a clear improvement on the results of iGEM Groningen 2014 mentioned above. Leakage experiments have demonstrated that bacteria not only survive, but also stay inside the gel. With this combination of survival and containment, we have made a prototype living material. In addition, we confirmed that the hydrogel is non-toxic, which is crucial for a wound healing application. In experiments on S. aureus performed by our collaborator PAMM, we observed that our lysostaphin constructs effectively kill (methicillin-resistant) S. aureus.
Our solution in practice
Approval
To allow the use of our living material as a wound healing plaster, we will need to prove its biocompatibility. We have demonstrated that our hydrogel does not contain any toxins after dialysis. Dextran is a biocampatible hydrogel, which is confirmed by its approval by the US Food and Drug Administration (FDA) and by the European Directorate for the Quality of Medicines (EDQM),(29). The E. coli strain used is safe to humans (30–32). Animal studies indicate that lysostaphin is non-toxic (12). This has, however, not been confirmed on humans. In principle, the components of our system are thus biocompatible, but the safety of the complete system must be verified in order to have our plaster approved as a medical device. As we learned from Koen Lim from Plasmacure, the approval trajectory will be a long and winding one. In our case, the approval trajectory would be even more complicated than for ‘regular’ pharmaceutics, as we are using living GMOs. For the remainder of the evaluation, we assume that the evaluation will succeed.
Costs
It is difficult to provide a cost estimation, as the final costs will be highly dependent on labor costs and the available manufacturing facilities. Nevertheless, we estimated the material costs. The production of one hydrogel (400 μL) only requires small dextran quantities (100 μg). Therefore, the estimated dextran costs for making one hydrogel in patch form is around €0,65. Adding other reagents will bring the total costs to approximately €1,00 for one hydrogel in small scale conditions. These costs can be optimized by upscaling.
Modification of the bacteria is a one-time expenditure. Afterwards they can be cultured and re-used for a long period. Culturing the bacteria can be performed on a large scale. The costs of medium are very low. The bacteria entrapped in the hydrogel can be modified to obtain different functionalities, such as the secretion of lysostaphin. Overall, the material costs for our living material are manageable, and upscaling will further reduce the costs. Although the exact manufacturing costs are unsure, a wound treatment with our patch should not be more expensive than the current costs of €7200 per leg ulcer (7).
Use
Stakeholder contributions resulted in some practical design requirements. Our plaster will be easy to use, as it can be simply applied directly on the skin and attached with some tape or a loose dressing. The patch can be easily sterilized with radiation, after which it can be delivered in a sterile wrapper. It can also be freeze-dried, which eases storage and transport. These properties ensure that even patients in remote places without well-equipped clinics can benefit from our product.
We will need to experimentally determine the optimal treatment duration. Previous research on bone fractures suggests that a week would be plenty for lysostaphin to eradicate E. coli (18). However, experts warned that bacteria may return if the wound is not yet closed. Dr. Edgar Peters predicted that the total treatment time will be several weeks or even months, during which the plaster must be replaced several times to prevent inflammation around the plaster.
Evaluation of our solution
We have demonstrated that our bandage is able to safely contain viable E. coli. Furthermore, we showed the production and secretion of functional lysostaphin which kills S. aureus. We expect to obtain a living material able to inhibit S. aureus if we combine both systems. This will allow us to develop a wound healing plaster. Here, we evaluate to which extent our bandage will provide a solution to chronic wounds and its implications for wound healing.
An essential design requirement was specificity. Patient Karel mentioned that the worst thing of having chronic wounds was the sustained treatment with systemic antibiotics, which gave him many side effects. Lysostaphin specifically and effectively targets Staphylococcus aureus. It is thus much more specific than most antibiotics. As the patch is applied locally, only bacteria on the wound should be targeted. Hopefully, our patch will thus improve patients’ quality of life. Patient suffering will be further reduced as our patch can stay on the wound for several days or perhaps weeks thanks to the sustained release provided by living bacteria. This will be an improvement particularly for burn wound patients, for whom the (current) daily wound dressing changes are painful.
Koen Lim from Plasmacure mentioned that the high costs of wound care are a burden for insurance companies. It is thus important to keep our solution affordable. Although not accurate, our cost calculation above indicates that the plaster will be cost-friendly. In addition, the reduced number of wound dressing changes will lower labor costs. The affordability of our system will not only be appealing to developed countries, but also to developing countries. As the plaster material is well-storable and transportable, it may provide an equal solution for patients in remote places and cities.
To prevent resistance against lysostaphin, we could consider a multi-angle approach inspired on the treatment of tuberculosis and malaria. For these diseases, the simultaneous administration of several drugs has been proven effective in the fight against resistance (34). Similarly, there is preliminary evidence that tolerance to a certain bacteriocin renders bacteria more susceptible to other toxins. Furthermore, experiments indicate that the combined activity of several bacteriocins is higher than the activity of each of them alone and that doses could be lowered (35). It has been observed that lysostaphin-resistant strains of S. aureus show increased susceptibility to methicillin. Methicillin-resistant strains were even rendered methicillin-sensitive (20). We may thus co-secrete lysostaphin with other bacteriocins or antibiotics.
Finally, the lysostaphin secretion results have positive implications on their own. Secretion significantly simplifies the purification and downstream processing of proteins (36). We established secretion without the use of any special equipment not present in a modest laboratory. This means that scientists, pharmacists and medical doctors in developing and developed countries alike may benefit from our method. They may thus use lysostaphin to treat patients without excessive costs.
Outlook
The first step towards a wound healing plaster would be the assembly of our secretion and adhesin systems. Based on stakeholder-feedback, we already designed some next steps to improve our design.
Additional bacteriocins
We want to expand on the secretion system with other bacteriocins, implement a sensing system, and we consider the implementation of a kill switch. Initially, we searched for a bacteriocin against Staphylococcus aureus. After feedback from several experts, we realized we should expand our secretion system with bacteriocins which target Pseudonomas aeruginosa, Streptococcus and preferably also other bacteria present on wounds. This is because wounds are always colonized by several bacterial strains. The eradication of S. aureus is an important first step towards wound healing, but may lead to other bacteria becoming more dominant in the wound flora. We propose to combine the expansion of our secretion system with sensing-based regulation to optimize the efficacy and minimize the chances of developing resistance against one or more of the bacteriocins.
Besides S. aureus, Pseudomonas and Enterococcus faecalis are generally reported as common bacteria on wounds (8). The Dutch experts we interacted with reported Pseudomonas as an important second bacterial strain, and one mentioned Streptococci. Other bacteria found on wounds are Proteus spp., coagulase-negative Staphylococci and anaerobic bacteria. Most ulcers are infected by more than one species (37).
We already designed a BioBrick to target P. aeruginosa. Pyocin S5 has been shown to be effective against mural lung infections (38,39). Ultimately, we will also equip E. coli with bacteriocins against Streptococcus and other pathogens. To target Streptococci, we may use a BioBrick from the registry. Mutacin III inhibits a range of gram-positive bacteria and has known effectivity against Streptococci. Gram-negative bacteria are immune, meaning that mutacin III is suitable for production by E. coli (40). Team UCL 2016 even developed an entire biosynthetic BioBrick for the production of mutacin III.
Integrated quorum sensor
Compared to common antibiotics, few examples of resistance against bacteriocins have been reported. The resistance frequencies differ greatly per bacteriocin (16). Importantly, resistance against lysostaphin is not often seen and there are indications that it comes with reduced fitness and increased susceptibility to other antibiotics (20). A murine study showed that pyocin S5 remained effective against P. aeruginosa, without the occurrence of resistance. However, one isolate became more tolerant to pyocin S5, indicating that the development of pyocin resistance is not completely unlikely (38). To decrease the chance of developing bacteriocin-immunity, we plan on regulating bacteriocin secretion with a sensing system. This avoids the needless use of bacteriocins.
Reducing the risk of resistance is not the only argument for a sensing system. The experts and stakeholders we interacted with provide two additional reasons: adaptation to a changing wound flora and ensuring that the correct medication is delivered from the start of the treatment onwards. Dr. Peters from the VU medical center expects that as soon as the dominating bacterial strain is exterminated, other bacteria may become dominant. Thus, to effectively and permanently sterilize a wound, continuous adaptation of the medication may be vital. This would be achieved through the integration of a quorum sensing construct which regulates the production and secretion of a variety of bacteriocins.
A second argument for the integration of sensor, was provided by the burn wound specialists we talked to in the Maasstad Hospital burn wound center. Current diagnostic techniques require cultivation of a wound sample. In the approximately four days taken by the diagnostic process, severe burn wounds are treated with systemic antibiotics. This procedure contributes to the development of antibiotic resistance, and, worse, is not always effective. By equipping our gel with a sensing system and a wide variety of bacteriocins against bacteria commonly found on (burn) wounds, we can avoid the diagnostics period. Instead, we will produce the appropriate medication on the spot, tailored to the patient, before a medical doctor could have made the correct diagnosis. This way, we make optimal use of the living bacteria we integrate into our hydrogel. Eventually, quorum sensing may also be adopted to prevent overcrowding of the gel and infiltration by foreign bacteria.
To regulate the production and secretion of our bacteriocins, we may use quorum sensing (QS). This is a microbial communication system based on chemical signaling pathways essential for survival in hostile environments. Intercellular signaling pathways regulate metabolism, growth, biofilm formation and other essential functions. Once reaching a biochemical threshold, a quorum sensing cascade may be activated, which regulates the activity of certain genes (42). We will benefit from QS pathways by regulating each effector gene by a quorum sensing construct.
Below we propose two example QS systems we could use as inspiration to regulate the secretion of lysostaphin and pyocin S5. Before implementing them, we will however need to review two aspects. Generally, when testing a QS-system, we must be aware of crosstalk. When different bacterial strains establish a co-culture, their QS systems may intervene. Receptors may be sensitive not only to the targeted ligand, but also to ligands of closely related strains (43). It will thus be important to check our sensing system only responds to the targeted malignant strains, and not to related benign strains.
Furthermore, we may want to consider the use of an irreversibly activated promotor or a transient promotor with a long persistency. Usually, QS-regulated promotors get activated once the ligand threshold is reached (43). This will start the production and secretion of the required bacteriocin. Subsequently, the pathogen concentration should drop. If we do not implement a waiting time, the transcription may be stopped once the concentration falls below the threshold. This may be dangerous as some surviving pathogens could still be present. These surviving bacteria may proliferate in the absence of bacteriocins, contributing to the growth of bacteriocin-resistant species. Click on the link below if you want to read about the sample quorum sensor systems for S. aureus and P. aeruginosa.
Quorum sensing systems for S. aureus and P. aeruginosa
We found quorum sensing systems developed by previous iGEM teams and other scientists which may be used to regulate the secretion of S. aureus and P. aeruginosa. As explained above, we would need to adapt them to ensure that the lysostaphin production is not switched off rapidly once the pathogen concentration drops below the threshold. Once we have expanded our bacteriocin collection, we should pair them with suitable QS-regulated systems.
Regulation of lysostaphin
To sense the presence of S. aureus, we may employ the accessory gene regulator (agr) QS system. S. aureus uses this system to switch from an attachment and colonization state at low cell density to the secretion of toxins and proteases at higher cell density (44). The system uses a kinase-response regulator pair (AgrA and AgrC) and an autoinducing peptide (AIP) AgrD. Upon binding of the AIP to AgrC, AgrA gets phosphorylated. Phosphorylated AgrA acts as an inducer to regulatory RNA. Amongst others, it regulates the production of AgrABCD. AgrB transports AgrD to the extracellular space to allow it to act as an autoinducer (44). Several iGEM teams designed constructs based on the agr system. We could for instance replace the pBAD promotor with BBa_I746104 , an AIP-inducible promotor P2 designed by iGEM Cambridge 2007 (figure 6).

Figure 6. Quorum sensing system to detect S. aureus as designed by team Cambridge 2007. It consists of AgrA, AgrB, AgrC and AgrD. Upon binding of AIP to AgrC, AgrA gets phosphorylated. AgrA-P activates the transcription of several regulatory genes by activating the P2 promotor, among which agrABCD. Reprinted from iGEM Cambridge 2007. Their image was based on Waters & Bassler (44).
Regulation of pyocin S5
To regulate the secretion of pyocin S5, we may develop a BioBrick based on a pathway designed by Saeidi et al. (45). They designed a synthetic genetic circuit for E. coli to sense P. aeruginosa , produce pyocin S5 and to lyse E. coli for the release of pyocin. Detection of P. aeruginosa is based on acyl homoserine lactones (AHLs), autoinducers uniquely produced by specific gram-negative strains. The lasR transcriptional factor regulated by a constitutive promotor binds AHL 3OC12HSL to form an activator complex for the PluxR promotors regulating pyocin S5 production and the production of cell lysis protein E7. Once the threshold concentration for E7 is reached, E. coli is lysed, releasing the accumulated pyocin into the extracellular environment. This inhibits P. aeruginosa . Thanks to our secretion tag, we will not need the cell lysis part of the circuit. Instead, we could also equip our secretion system HlyB/D with a PluxR promotor (figure 7).
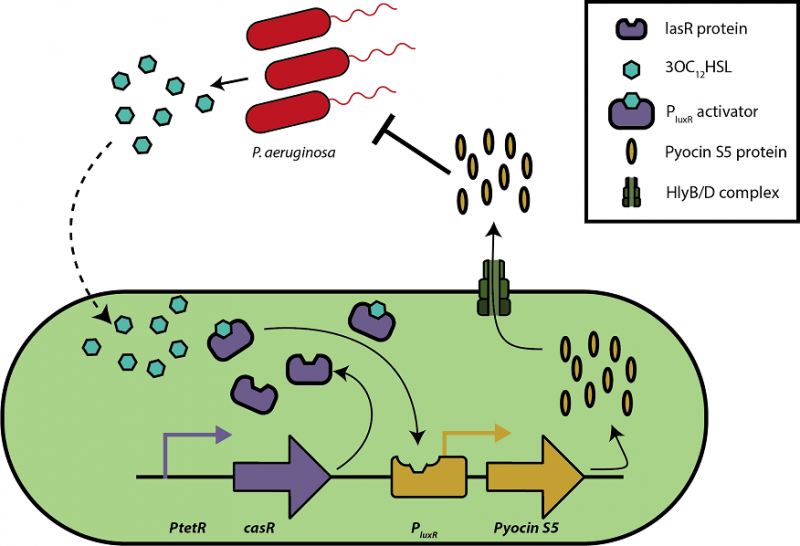
Figure 7. A quorum sensing-regulated pyocin secretion circuit adapted from the circuit developed by Saedi et al. (45). Upon detection of AHL 3OC12 HSL specifically produced by P. aeruginosa , lasR form an activator complex with 3OC12 HSL. It activates the PluxR promotor which ensures the production of pyocin S5. Subsequently, pyocin S5 is secreted by E. coli’s Type I secretion system (through the HlyB/D complex) and inhibits P. aeruginosa .
Kill switch
To further improve the biosafety of Gelcatraz, we considered the use of a kill switch. If bacterial leakage happens despite the adhesin system, the kill switch will ensure that any escaped bacteria die. This will prevent the dispersion of modified DNA. The dispersion of modified DNA is not desirable, since it can have unpredictable consequences on its own or after mutation several generations later (47–50).
After reviewing several kill switch systems by the group of J.J. Collins at Massachusetts Institute of Technology (47) and the group of G.M. Church at Harvard Medical School in Boston (51) and others, we decided that one of two kill switches designed by the group of P.A. Silver at Harvard University and Harvard Medical School in Boston (52) would be a great addition to our project to restrain function of our GMOs outside of the dextran hydrogel. In contrast to the kill switch proposed by professor Jan van Hest, these kill switches do not rely on artificial amino acids. This makes them significantly cheaper (53).
Stirling et al. describe the type II toxin-antitoxin system CcdA and CcdB (52). CcdA is the antitoxin of CcdB, which can be inhibited by direct binding to CcdA. The CcdB protein is toxic to E. coli because it inhibits a part of DNA gyrase (a type II topoisomerase), which is essential for survival.
Stirling et al. include a bistable switch system of bacteriophage lambda in this toxin-antitoxin system consisting of proteins cI and Cro. The combination of a simple toxin-antitoxin system with this switch functioning as a memory element increases the amount of bacterial generations the switch survives before being shut down by any kind of DNA modification. They experimentally showed that several candidate strains of bacteria could retain their kill switch system for approximately 140 generations (48).
This retainment is very impressive and would be an interesting addition to our engineered E. coli. The lengths to which the kill switch remains active enables our E. coli to have a reliable system for several days. This time span comes close to the period we want to realistically apply our patch on wounds for.
The future of our living material
In our iGEM project, we demonstrated our living material as a useful platform for wound healing. However, we envision our living material to be a much broader platform useful for a range of applications. Living materials have the potential to perform extraordinary functions. We invite future iGEM teams to use our platform for their own creative applications. Team Hamburg has already investigated our hydrogel for their own project. We hope that more applications will follow!
Acknowledgements
The design of the living material and the wound patch has been informed by interactions with several stakeholders. We described these interactions on the integrated human practices page. However, we would like to once again thank all of them for their valuable input. Our project would not have been the same without you!
We want to thank the Dutch National Institute for Public Health and the Environment (RIVM), the Dutch Institute for Technology Assessment (RIVM), PAMM, Maasstad Hospital Burn Wound Center, Plasmacure, Expertise Center Wound Care, chronic wound patient Karel, Dr. Edgar Peters (VUmc), Prof. Dr. Menno Prins (TU/e), Prof. Dr. Jan van Hest (TU/e), Prof. Dr. Patricia Dankers (TU/e), Dr. Lily Frank (TU/e), Dr. Sandra Hofmann (TU/e), Lesley Bouwer, M.D., iGEM Düsseldorf 2018 and finally the scientists and professionals we met at the Netherlands Biotechnology Congress 2018.
References
We derived a lot of information on burn wounds and (diabetic) chronic wound infections from interviews with medical doctors from the Maasstad Hospital, VUmc (Edgar Peters), Expertise Center Wound Care and a visit to the start-up company Plasmacure. In addition, we extensively used the scientific literature to verify our observations.- Liu X, Tang T-C, Tham E, Yuk H, Lin S, Lu TK, et al. Stretchable living materials and devices with hydrogel–elastomer hybrids hosting programmed cells. Proc Natl Acad Sci [Internet]. 2017;114(9):2200–5. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.1618307114
- Cheng AA, Lu TK. Synthetic Biology: An Emerging Engineering Discipline. Annu Rev Biomed Eng [Internet]. 2012;14(1):155–78. Available from: http://www.annualreviews.org/doi/10.1146/annurev-bioeng-071811-150118
- Gerber LC, Koehler FM, Grass RN, Stark WJ. Incorporation of penicillin-producing fungi into living materials to provide chemically active and antibiotic-releasing surfaces. Angew Chemie - Int Ed. 2012;51(45):11293–6.
- Capgemini Consulting. Innovatie van complexe wondzorg. Onderzoek naar potentiële besparingen en prestatieomschrijvingen. Innov van complexe wondzorg. 2014;55.
- Serra R, Grande R, Butrico L, Rossi A, Settimio UF, Caroleo B, et al. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther. 2015;13(5):605–13.
- Meerwaldt R, Das F, Fentener van Vlissingen J, de Lange E, Maessen-Visch MB, van Montfrans C, et al. Kwaliteitsstandaard Organisatie van wondzorg in Nederland [Internet]. 2018. Available from: https://www.demedischspecialist.nl/sites/default/files/Kwaliteitsstandaard Organisatie van wondzorg in Nederland.pdf
- Meerwaldt R, Das F, Fentener van Vlissingen J, de Lange E, Maessen-Visch MB, van Montfrans C, et al. Concept- kwaliteitsstandaard complexe wondzorg.
- Gjødsbøl K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, Krogfelt KA. Multiple bacteria I species reside in chronic wounds : a longitudinal study. 2006;3(3):225–31.
- Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T A peer-reviewed J Formul Manag [Internet]. 2015;40(4):277–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25859123%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4378521%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/25859123%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4378521
- Gross M. Antibiotics in crisis. Curr Biol [Internet]. 2013;23(24):R1063–5. Available from: http://dx.doi.org/10.1016/j.cub.2013.11.057
- Rossolini GM, Arena F, Pecile P, Pollini S. Update on the antibiotic resistance crisis. Curr Opin Pharmacol [Internet]. 2014;18:56–60. Available from: http://dx.doi.org/10.1016/j.coph.2014.09.006
- Bastos M do C de F, Coutinho BG, Coelho MLV. Lysostaphin: A staphylococcal bacteriolysin with potential clinical applications. Pharmaceuticals. 2010;3(4):1139–61.
- Mullard A. Momentum builds around new antibiotic business models. Nat Rev Drug Discov [Internet]. 2014;13(10):711–3. Available from: http://dx.doi.org/10.1038/nrd4455
- Ochsendorf F. Systemic antibiotic therapy of acne vulgaris. Jddg [Internet]. 2006;4(10):828–41. Available from: http://doi.wiley.com/10.1111/j.1610-0387.2006.06053.x
- Balciunas EM, Castillo Martinez FA, Todorov SD, Franco BDG de M, Converti A, Oliveira RP de S. Novel biotechnological applications of bacteriocins: A review. Food Control [Internet]. 2013;32(1):134–42. Available from: http://dx.doi.org/10.1016/j.foodcont.2012.11.025
- Kjos M, Borrero J, Opsata M, Birri DJ, Holo H, Cintas LM, et al. Target recognition, resistance, immunity and genome mining of class II bacteriocins from Gram-positive bacteria. Microbiology. 2011;157(12):3256–67.
- De Kwaadsteniet M, Doeschate KT, Dicks LMT. Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus. Lett Appl Microbiol. 2009;48(1):65–70.
- Johnson CT, Wroe JA, Agarwal R, Martin KE, Guldberg RE, Donlan RM, et al. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc Natl Acad Sci U S A [Internet]. 2018;(12):201801013. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29760099
- Szweda P, Gorczyca G, Filipkowski P, Zalewska M, Milewski S. Efficient production of Staphylococcus simulans lysostaphin in a benchtop bioreactor by recombinant Escherichia coli. Prep Biochem Biotechnol. 2014;44(4):370–81.
- Kusuma C, Jadanova A, Chanturiya T, Kokai-Kun JF. Lysostaphin-resistant variants of Staphylococcus aureus demonstrate reduced fitness in vitro and in vivo. Antimicrob Agents Chemother. 2007;51(2):475–82.
- Saul JM, Williams DF. Hydrogels in Regenerative Medicine. Handb Polym Appl Med Med Devices. 2013;279–302.
- Liu X, Yuk H, Lin S, Parada GA, Tang TC, Tham E, et al. 3D Printing of Living Responsive Materials and Devices. Adv Mater. 2018;30(4):1–9.
- Mora CA, Herzog AF, Raso RA, Stark WJ. Programmable living material containing reporter micro-organisms permits quantitative detection of oligosaccharides. Biomaterials [Internet]. 2015;61:1–9. Available from: http://dx.doi.org/10.1016/j.biomaterials.2015.04.054
- Gerber LC, Koehler FM, Grass RN, Stark WJ. Incorporating microorganisms into polymer layers provides bioinspired functional living materials. Proc Natl Acad Sci [Internet]. 2012;109(1):90–4. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.1115381109
- Guo S, Stevens CA, Vance TDR, Olijve LLC, Graham LA, Campbell RL, et al. Structure of a 1.5-MDa adhesin that binds its Antarctic bacterium to diatoms and ice. Sci Adv. 2017;3(8).
- Plieva F, Oknianska A, Degerman E, Galaev IY, Mattiasson B. Novel supermacroporous dextran gels. J Biomater Sci Polym Ed. 2006;17(10):1075–92.
- Lévesque SG, Lim RM, Shoichet MS. Macroporous interconnected dextran scaffolds of controlled porosity for tissue-engineering applications. Biomaterials. 2005;26(35):7436–46.
- Szafulera K, Wach RA, Olejnik AK, Rosiak JM, Ulański P. Radiation synthesis of biocompatible hydrogels of dextran methacrylate. Radiat Phys Chem. 2018;142(October 2016):115–20.
- Pharmacosmos. Pharmaceutical Quality Dextran [Internet]. Available from: https://www.dextran.com/category?catalog=24&category=70
- New England BioLabs. Safety Data Sheet: BL21(DE3) Competent E.coli. 2016. p. 1–9.
- Merck. BLR(DE3) Competent Cells - Novagen [Internet]. Available from: https://www.merckmillipore.com/NL/en/product/BLRDE3-Competent-Cells-Novagen,EMD_BIO-69053?ReferrerURL=https%3A%2F%2Fwww.google.nl%2F
- Ministry of Social Affairs and Employment. Wetgeving biologische agentia [Internet]. [cited 2018 Oct 3]. Available from: https://www.arboportaal.nl/onderwerpen/wetgeving-biologische-agentia
- Chung H, Kim TY, Lee SY. Recent advances in production of recombinant spider silk proteins. Curr Opin Biotechnol [Internet]. 2012;23(6):957–64. Available from: http://dx.doi.org/10.1016/j.copbio.2012.03.013
- Bloland PB, Ettling M. Making malaria-treatment policy in the face of drug resistance. Ann Trop Med Parasitol. 1999;93(1):5–23.
- de Freire Bastos MDC, Varella Coelho ML, da Silva Santos OC. Resistance to bacteriocins produced by gram-positive bacteria. Microbiol (United Kingdom). 2015;161(4):683–700.
- Mergulhão FJM, Summers DK, Monteiro GA. Recombinant protein secretion in Escherichia coli. Biotechnol Adv. 2005;23(3):177–202.
- Siddiqui AR, Bernstein JM. Chronic wound infection: Facts and controversies. Clin Dermatol [Internet]. 2010;28(5):519–26. Available from: http://dx.doi.org/10.1016/j.clindermatol.2010.03.009
- McCaughey LC, Ritchie ND, Douce GR, Evans TJ, Walker D. Efficacy of species-specific protein antibiotics in a murine model of acute Pseudomonas aeruginosa lung infection. Sci Rep [Internet]. 2016;6(June):1–8. Available from: http://dx.doi.org/10.1038/srep30201
- Elfarash A, Dingemans J, Ye L, Hassan AA, Craggs M, Reimmann C, et al. Pore-forming pyocin S5 utilizes the FptA ferripyochelin receptor to kill Pseudomonas aeruginosa. Microbiol (United Kingdom). 2014;160(PART 2):261–9.
- Hillman JD, Johnson KP, Yaphe BI. Isolation of a Streptococcus mutans strain producing a novel bacteriocin. Infect Immun. 1984;44(1):141–4.
- Draper LA, Cotter PD, Hill C, Ross RP. Lantibiotic Resistance. Microbiol Mol Biol Rev [Internet]. 2015;79(2):171–91. Available from: http://mmbr.asm.org/lookup/doi/10.1128/MMBR.00051-14
- Asad S, Opal SM. Bench-to-bedside review: Quorum sensing and the role of cell-to-cell communication during invasive bacterial infection. Crit Care [Internet]. 2008;12(6):236. Available from: http://ccforum.biomedcentral.com/articles/10.1186/cc7101
- Scott SR, Hasty J. Quorum Sensing Communication Modules for Microbial Consortia Graphical Abstract HHS Public Access. ACS Synth Biol [Internet]. 2016;5(9):969–77. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5603278/pdf/nihms886986.pdf
- Waters CM, Bassler BL. QUORUM SENSING: Cell-to-Cell Communication in Bacteria. Annu Rev Cell Dev Biol [Internet]. 2005;21(1):319–46. Available from: http://www.annualreviews.org/doi/10.1146/annurev.cellbio.21.012704.131001
- Saeidi N, Wong CK, Lo TM, Nguyen HX, Ling H, Leong SSJ, et al. Engineering microbes to sense and eradicate Pseudomonas aeruginosa, a human pathogen. Mol Syst Biol [Internet]. 2011;7(521):1–11. Available from: http://dx.doi.org/10.1038/msb.2011.55
- iGEM Groningen 2014. Detection of infected Wounds [Internet]. 2014. Available from: https://2014.igem.org/Team:Groningen:Project:Detection
- Chan CTY, Lee JW, Cameron DE, Bashor CJ, Collins JJ. “Deadman” and “Passcode” microbial kill switches for bacterial containment. Nat Chem Biol. 2016;12(2):82–6.
- Stirling F, Bitzan L, O’Keefe S, Redfield E, Oliver JWK, Way J, et al. Rational Design of Evolutionarily Stable Microbial Kill Switches. Mol Cell [Internet]. 2017;68(4):686–96. Available from: http://dx.doi.org/10.1016/j.molcel.2017.10.033
- Caliando BJ, Voigt CA. Targeted DNA degradation using a CRISPR device stably carried in the host genome. Nat Commun [Internet]. 2015;6(May):1–10. Available from: http://dx.doi.org/10.1038/ncomms7989
- Mandell DJ, Lajoie MJ, Mee MT, Takeuchi R, Kuznetsov G, Norville JE, et al. Biocontainment of genetically modified organisms by synthetic protein design. Nature [Internet]. 2015;518(7537):55–60. Available from: http://dx.doi.org/10.1038/nature14121
- Mandell DJ, Lajoie MJ, Mee MT, Takeuchi R, Kuznetsov G, Norville JE, et al. Biocontainment of genetically modified organisms by synthetic protein design. Nature. 2015;518(7537):55–60.
- Stirling F, Bitzan L, O’Keefe S, Redfield E, Oliver JWK, Way J, et al. Rational Design of Evolutionarily Stable Microbial Kill Switches. Mol Cell. 2017;68(4):686–96.
- Mándity IM, Olasz B, Ötvös SB, Fülöp F. Continuous-flow solid-phase peptide synthesis: A revolutionary reduction of the amino acid excess. ChemSusChem. 2014;7(11):3172–6.