HANSONGTSENG (Talk | contribs) |
HANSONGTSENG (Talk | contribs) |
||
| Line 65: | Line 65: | ||
<h2>DKK1 FRET Detection System</h2> | <h2>DKK1 FRET Detection System</h2> | ||
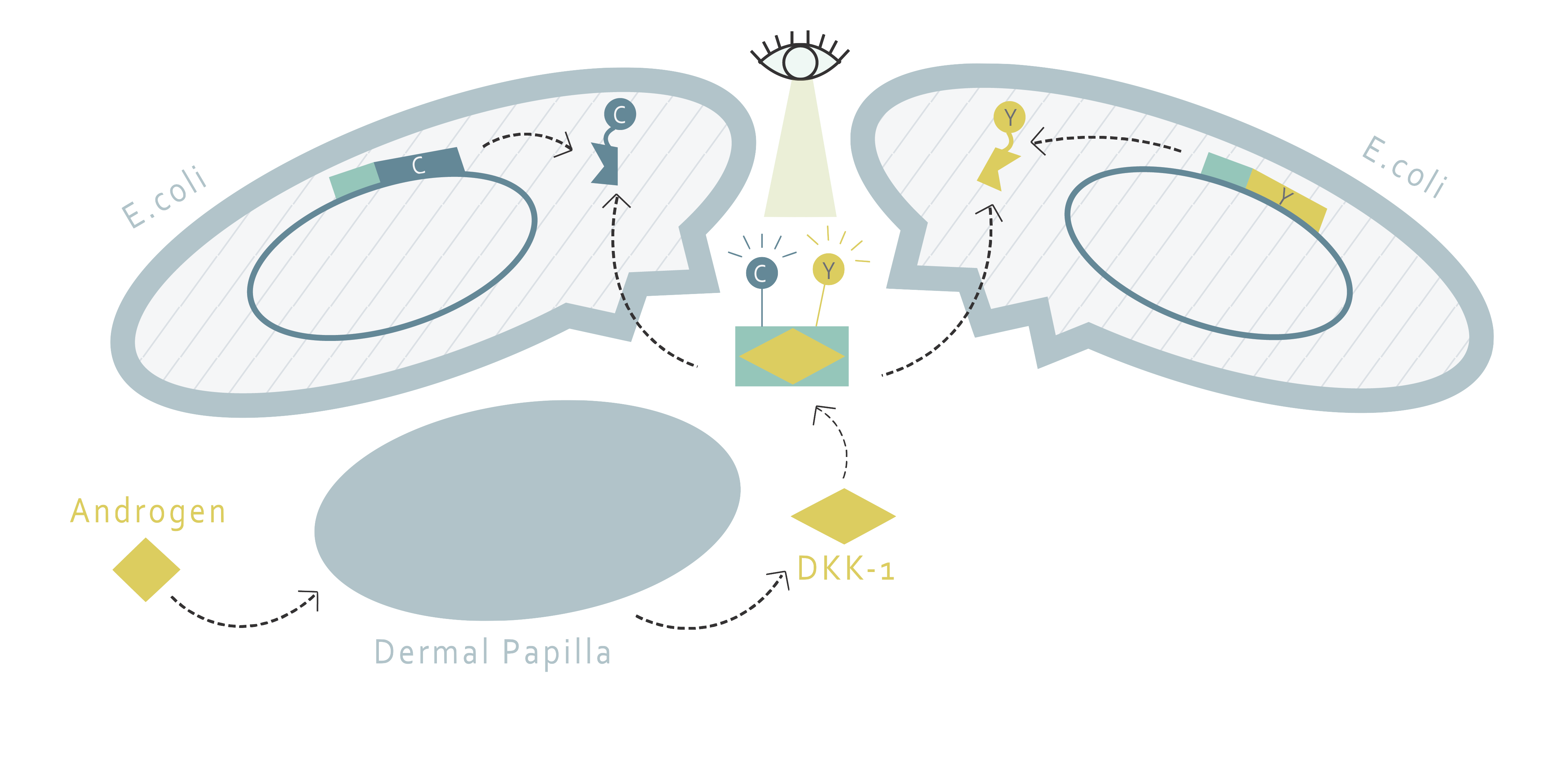
<p>By fusing DKK1 binding proteins and FRET pairs together, we were able to create a DKK1 detection system using FRET. When DKK1 exists in the solution, two types of fusion proteins can be added to determine its existence. The two proteins would both bind to DKK1. As a DKK1 is attached by both of them, the FRET pairs on the fusion proteins would get close enough to induce FRET reaction. We can use plate readers to measure the fluorescence pattern to determine whether FRET has occurred, thereby estimating the concentration of DKK1.</p> | <p>By fusing DKK1 binding proteins and FRET pairs together, we were able to create a DKK1 detection system using FRET. When DKK1 exists in the solution, two types of fusion proteins can be added to determine its existence. The two proteins would both bind to DKK1. As a DKK1 is attached by both of them, the FRET pairs on the fusion proteins would get close enough to induce FRET reaction. We can use plate readers to measure the fluorescence pattern to determine whether FRET has occurred, thereby estimating the concentration of DKK1.</p> | ||
| − | <img src=""> | + | <img src="https://static.igem.org/mediawiki/2018/e/e1/T--NYMU-Taipei--project-description-solution3.png"> |
</div> | </div> | ||
</div> | </div> | ||
Revision as of 02:13, 18 October 2018

FPF system
Success in constructing a FRET Protein Fusion(FPF) system
To simplify the construction of FRET fusion proteins, we have designed a system to standardize the process. The system consists of two plasmids-- pET32a FPF-skel1 and pET32a FPF-skel2.
pET32a FPF-skel1 is a pET32a whose original MCS is replaced with our newly designed MCS -- FPF-Skel1. This new MCS allows one fluorescent protein and another protein(in our case, a DKK1-binding protein)to be inserted. The inserted proteins would be expressed as fusion protein by a T7 promoter with fluorescent protein attached to the C-terminal of the other protein and his tag attached to the C-terminal of fluorescent protein(called Binding-Fluorescent type, BF type).

pET32a FPF-skel2 functions similarly. However, in the protein expressed by this plasmid, the fluorescent protein is attached to the N-terminal of the other protein and his tag is attached to the N-terminal of the fluorescent protein(called Fluorescent-Binding type, FB type). In this case, the sequence of protein is inverted.

We made this additional plasmid because FRET efficiency is highly related to the distance between the FRET pairs. Having one of the FRET pair at the farther end of a single domain of a protein, instead of the closer end, might result in drastic difference in FRET efficiency. It is also worth mentioning that modeling protein conformation might still be different from reality. Hence, to make sure that we can find the best protein conformation for FRET, we intend to express both BF and FB type fusion protein.
With the FRET protein fusion system, we are able to construct plasmids below with ease. That the backbone’s effect on protein conformation has also been confirmed to be minimal as it contains little unnecessary sequence and the expressed proteins has functioned normally as can be seen in fluorescence graphs below.
- pET32a FPF-Skel1 YPet-Ubc9
- pET32a FPF-Skel1 YPet-H7
- pET32a FPF-Skel1 YPet-E1E2
- pET32a FPF-Skel1 YPet-E3E4
- pET32a FPF-Skel1 YPet-E1
- pET32a FPF-Skel1 YPet-E3
- pET32a FPF-Skel1 CyPet-SUMO1
- pET32a FPF-Skel1 CyPet-VHH G5
- pET32a FPF-Skel1 CyPet-VHH H7
- pET32a FPF-Skel1 CyPet-E3E4
- pET32a FPF-Skel1 CyPet-E3


FRETCheck
FRET Protein Checking System
In developing our project, we created a system to check wether our fluorescent proteins can FRET with each other. we attached one of the fret pairs to SUMO1 and the other to Ubc9. SUMO1 and Ubc9 are proteins that would automatically attach to each other, so the FRET pairs attached to them are supposed to get close enough to induce FRET reaction once the two type of fusion proteins are added together under proper condition. To verify the system we conducted the experiment with CyPet and YPet. CyPet and YPet has been attached to SUMO1 and Ubc9 respectively. The result is shown in FRET System experiments and results Exp5.
FRETDteKt
DKK1 FRET Detection System
By fusing DKK1 binding proteins and FRET pairs together, we were able to create a DKK1 detection system using FRET. When DKK1 exists in the solution, two types of fusion proteins can be added to determine its existence. The two proteins would both bind to DKK1. As a DKK1 is attached by both of them, the FRET pairs on the fusion proteins would get close enough to induce FRET reaction. We can use plate readers to measure the fluorescence pattern to determine whether FRET has occurred, thereby estimating the concentration of DKK1.
Cell Screening System
A Convenient Screening Platform for AGA Drugs Has Been Created
We created a drug screening platform with HEK 293T cell line, a commonly used kidney cancer cell that has relatively high expression of DKK1. We constructed a plasmid with human DKK1 promoter and an mCherry reporter gene. We have proven that the DKK1 promoter can be successfully activated in cells and the mCherry protein can be secreted. Moreover, the DKK1 promoter and mCherry protein can be stimulated with testosterone and DHT, which corresponds with our prediction and literature reviews . Suitable testosterone and DHT concentrations have also been optimized for the screening platform. Figure shown below is the schematic diagram of our system. (For more information, visit Experiments and Results: Exp5 and Exp6)

Secreting Reporter System
Introducing a mammalian secreting reporter system
We successfully tagged albumin secreting signal peptide (ALB) to fluorescence reporter proteins, resulting in the extracellular secretion of the fluorescence protein. This allows the reporter to be detected extracellularly via a plate reader. This design allows fast and convenient detection of the reporter system. We briefly summarize the advantage of using our mammalian secreting reporter system below:
- Cheaper compared to traditional reporters such as luciferase or SEAP protein
- Fast and Convenient
- Does less harm to the cultured cells and allows continuous observation
- Does not require high technical skills
(For more information, visit Improvement: ALB secretion peptide)

Entrepreneurship
Business Plans and Cooperation
We have a comprehensible business aim that composes into two main parts. Therefore, following our aims, we have a straightforward and effective business plan. We tried our best to engage in extensive outreach approaches including discussing with customers, competitors and patients. We also went to an art exhibition which we reflect our role of responsibility in the biomedical industry. We not only do marketing analysis but also understand the practical situation of the biomedical industries, in which, having a patent and comparing competitors’ patent information with ours could be the most essential part of our entrepreneurship. We also put a big effort into advertising our products. This has shown the viability and diversity of our product. Last but not least, our plans and actions really worked. We, therefore, raised a great amount of funding for the basis of a good start. The pictures shown below include our designed logo and our visit to companies. (For more information, visit Entrepreneurship)
Our Logo

Company Visits


