

This year team XMU-China developed cell-free systems to detect and treat diseases. Protein detection is unique and significant in biology fields, especially for the detection of protein biomarkers which produced by diseased cells. In order to overcome the deficiencies of traditional detection methods, we have developed an Aptamer Based Cell-free Detection system (ABCD system) of protein. The core of the ABCD system is the specific binding of the aptamer and its target protein. After protein detection, we use outer-membrane vesicles (OMVs) to treat the diseased cells. We designed a system that has realized the efficient, customizable production of OMVs, which serves to encapsulate specific siRNA for disease treatment. To guarantee the practicability detection and treatment system, we also improved KaiABC system and TDPs system to regulate the expression rate of OMVs and store fragile chemicals or biological materials.
In order to equip our idea with social demands, we have been practicing all through our project by conducting investigation on diseases detection and corresponding information, visiting biotechnology company to gain a better knowledge about the diseases detection, interacting with public to propagate synthetic biology and learning from teams applied themselves in diseases detection theme. We divided these activities in four stages which are Laws and Regulations, Inspiration and Reality, Public and Promotion, Idea and Wisdom, respectively. We have completed Human Practices silver medal criteria, Human Practices gold medal criteria/ Best Integrated Human Practices award and Best Education and Public Engagement award.

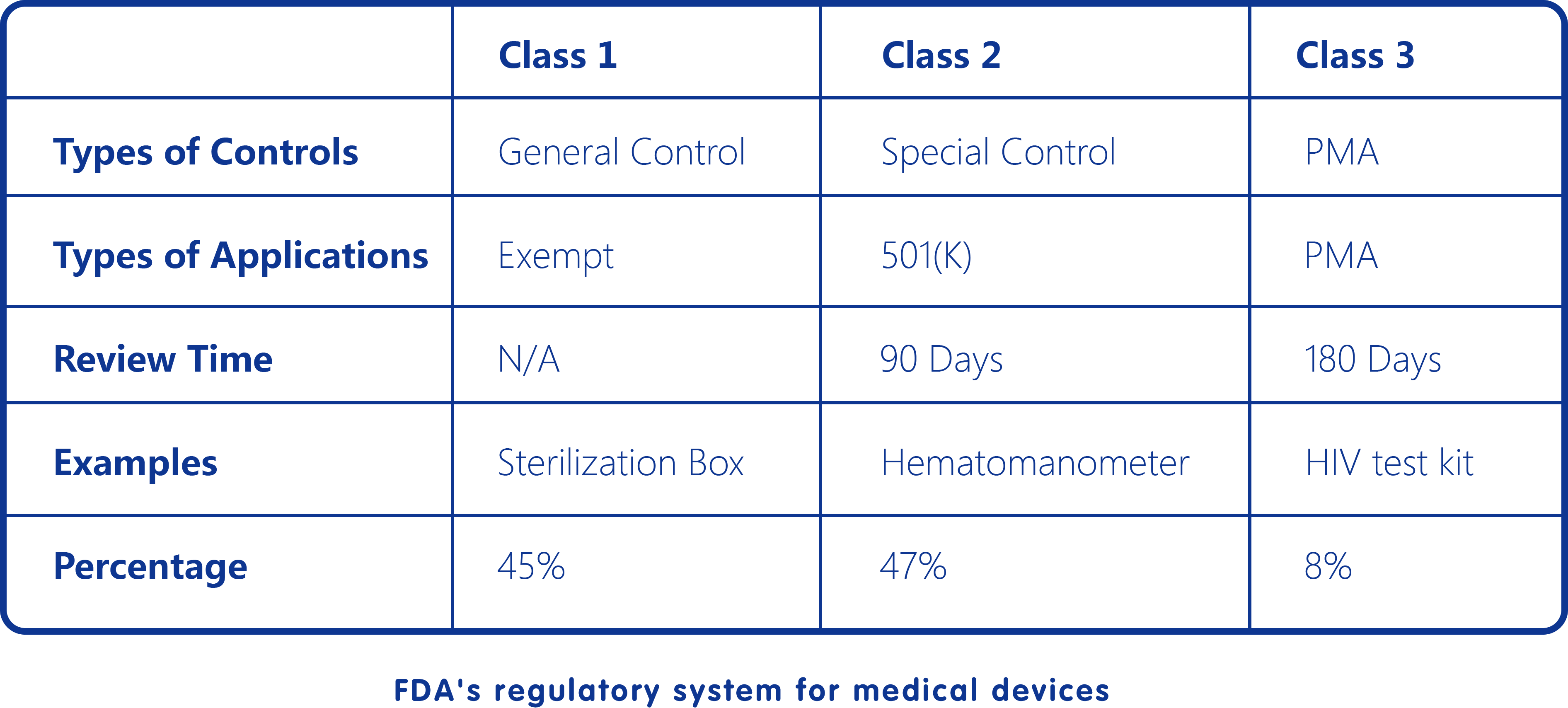
In addition,during human practice activities, we found that most disease-diagnosing methods are confined to specific delicate testing apparatus, which are expensive, time-consuming and low sensitivity. The study of Point-of-care testing (POCT), also called bedside testing (with the definition of medical diagnostic testing at or near the time and place of patient care), has become very heated because of its convenience, simplicity and highly efficiency. Internet of things (IoT) is the network of physical devices, vehicles, home appliances, and other items embedded with electronics, software, sensors, actuators, and connectivity which enables the connection and exchange of data.
Here we came up with a design. We combined the idea of our project-Aptamer-based Cell-free Detection system (ABCD system), IoT, and the above concept of POCT so as to develop a microfluidic device, which is small while convenient for real-time detection of cancer. To see the Hardware(硬件设计链接)and the Business plan(商业计划链接).
Creating a brainstorm is simplified, how to put theory into practice matters more. For understanding diseases detection reality socially, we visited three organizations, which all have authority background in Xiamen area. In order to consider the feasibility of the ABCD system in our project, we interviewed Professor Zhu Zhi who has very fruitful results on aptamers, the Chief Physician Weiwei Tang and Professor Zengfu Xue. We finally visited a grass-roots Health Service Center to find our product’s market positioning.
Visiting Mr. Su Mingluo, founder and chairman of Xiamen Kymem Membrane Technology Ltd. 2018.09.30 On September 30th 2018, we visited Mr. Su Mingluo, founder and chairman of Xiamen Kymem Membrane Technology Ltd, to have a deep discussion about information related to enterprises. He shared with us his experience of setting-up a new company, and rendered us prepared for drawing up business plans.
In the view of Mr. Su, having a sufficient knowledge about not only your company, but your competitors’ on the basis of market analysis was the premise of drawing up a good business plan. The business plan should involve some basic parts, namely the company’s technology introduction, market potential demand, market actual demand, team composition and competitive team analysis. We introduced our project, which joined competition this year, to Mr.Su, and highlighted the hardware products which we independently developed. Mr. Su expressed his expectation for the potential of our hardware equipment in the medical field. But on the other hand, given that starting a new business is tough, Mr. Su believed that our team would encounter five “new” threats in the initial stage, that is, new team, new cooperation partners, new capital, new technologies and new customers.
He also mentioned that at the initial period of our business, not only the company’s internal developing problems, but also the constrictions of the external environment would be the obstacle. Referring to his personal accumulated experience, Mr. Su further gave us some suggestions targeting on starting a new business: considering that China’s current intellectual property law system was still imperfect, innovative companies, like ours, who were known for their technological advantages must emphasize the patent-protection. What’s more, when our brand was still not so influential compared to those prestigious companies, we should put seeking target customers and broadening sales channels on the front burner, and carefully prevent our technology property right from being infringed. On top of that, he proposed that since our hardware product was based on the "government + grassroots + personal" operation mode, it was necessary to work hard in marketing, with clear targets and strategies.
On September 30th 2018, we visited Mr. Su Mingluo, founder and chairman of Xiamen Kymem Membrane Technology Ltd, to have a deep discussion about information related to enterprises. He shared with us his experience of setting-up a new company, and rendered us prepared for drawing up business plans.
On September 30th 2018, we visited Mr. Su Mingluo, founder and chairman of Xiamen Kymem Membrane Technology Ltd, to have a deep discussion about information related to enterprises. He shared with us his experience of setting-up a new company, and rendered us prepared for drawing up business plans.
In the view of Mr. Su, having a sufficient knowledge about not only your company, but your competitors’ on the basis of market analysis was the premise of drawing up a good business plan. The business plan should involve some basic parts, namely the company’s technology introduction, market potential demand, market actual demand, team composition and competitive team analysis. We introduced our project, which joined competition this year, to Mr. Su, and highlighted the hardware products which we independently developed. Mr. Su expressed his expectation for the potential of our hardware equipment in the medical field. But on the other hand, given that starting a new business is tough, Mr. Su believed that our team would encounter five “new” threats in the initial stage, that is, new team, new cooperation partners, new capital, new technologies and new customers.
He also mentioned that at the initial period of our business, not only the company’s internal developing problems, but also the constrictions of the external environment would be the obstacle. Referring to his personal accumulated experience, Mr. Su further gave us some suggestions targeting on starting a new business: considering that China’s current intellectual property law system was still imperfect, innovative companies, like ours, who were known for their technological advantages must emphasize the patent-protection. What’s more, when our brand was still not so influential compared to those prestigious companies, we should put seeking target customers and broadening sales channels on the front burner, and carefully prevent our technology property right from being infringed. On top of that, he proposed that since our hardware product was based on the "government + grassroots + personal" operation mode, it was necessary to work hard in marketing, with clear targets and strategies.

We visited Mr. Jianghong Wu, president of Xiamen Wenda technology LTD. We had an enjoyed communication with Mr. Wu, which is about the communication mode between team hardware and software and the practicability of software design. We introduced the general situation of this year's team project to Mr. Wu, and emphasized the original intention of the team to solve the "aerial phenomenon" of the current "graded diagnosis and treatment" policy, as well as the difficulty in getting medical treatment in remote areas. Mr. Wu fully affirmed the innovative project and praised the team consciousness of social responsibility.
As for APP part he gave some professional advices and technical guidance, like how to accelerate our convolution neural network recognition speed with collected increasing number of images cases, and how to link block them up between chain technology public health service database to serve the public better. In addition, Mr. Wu gave us a lot of advice on product promotion and profit model.

In order to consider the feasibility of the ABCD system in our project, we interviewed Professor Zhu Zhi of the College of Chemistry and Chemical Engineering of Xiamen University on May 2, and she has very fruitful results on aptamers.
The professor affirmed the feasibility of our project and gave us some invaluable advice. Under the suggestion of Professor Zhu Zhi, we chose EpCAM and aptamer SYL3C. In addition, we are very grateful to Professor Zhu for her support on many instruments and materials provided during our experiments.

IThis year,team XMU-China developed new methods named ABCD System and OMVs Treatment for detecting and treating diseases. In order to prove the feasibility of our project, we consulted the Chief Physician Weiwei Tang in Medical Oncology, The First Affiliated Hospital of Xiamen University, on August 23th. We introduced briefly the structure and function of our self-designed hardware Eye of Agamotto (EA) to Dr. Tang. During the talk, Dr. Tang appreciated it that our ideas are innovative and helpful. What’s more, Dr. Tang also told us those frequently-used and classical clinical detection methods, like ELISA, CLIA and so on. Most of which are time-consuming, expensive and complex, compared with EA. As for EA, Dr. Tang agreed with our proposals that we are going to make EA widely used among basic medical institutions, especially in remote, and poverty-stricken areas because of its convenient and practicability. But it’s worth noting that today there is an authoritative method for disease-diagnose, i.e., pathological diagnosis. So our detection method would just serve as a kind of screening tool rather than diagnosis method, according to Tang’s suggestion.
The professor affirmed the feasibility of our project and gave us some invaluable advice. Under the suggestion of Professor Zhu Zhi, we chose EpCAM and aptamer SYL3C. In addition, we are very grateful to Professor Zhu for her support on many instruments and materials provided during our experiments.

We visited Professor Zengfu Xue, deputy director of the Cancer Early Screening Diagnosis and Treatment Center, the First Affiliated Hospital of Xiamen University. We exchanged and discussed with professor Xue about the design of cell free detection system of tumor markers and detection carrier of microfluidic chip. Professor Xue fully affirmed the innovation and feasibility of this project, and appreciated the original intention of the team to solve the problem of difficult access to medical services in large hospitals, which helped to improve the current "hierarchical diagnosis and treatment" policy system to some extent. We communicated with professor Xue about the next clinical trial plan of the detection hardware Eye of Agamotto, and reached a preliminary cooperation agreement. If possible, we could take the instrument to the lab in hospital, and use the patient's blood sample to verify the performance of the instrument, such as stability and sensitivity.


We visited Health Service Center of Datong Street, Tong’an District, Xiamen city. We learned about the development of tumor markers detection work in primary hospitals from director Zhan. We found that primary hospitals were mostly able to carry out screening of basic tumor markers such as alpha fetoprotein. If people want to carry out more systematic and specific tumor screening, they had to be referred to higher-level hospitals. In addition, due to the implementation effect of China's medical security system and Xiamen's health policy, the screening of tumor markers at the grassroots-level health center could save about 50% of the cost compared with the large hospitals. Therefore, to sum up, president Zhan affirmed the future prospects of our instruments, believing it would solve problems such as "difficult access to medical services" in real life, to better provide more convenient medical and health services.

The medical reform policies, together with the reasonable superintendence will promote the rapid progress of POCT(point-of-care testing) in America.

America now is encountered with the payment pressures imposed by enormous medical insurance fees resulted from the aged tendency of population and the changes in the spectrum of diseases. According to the forecast by CBO, the expense of health products will reach up to 4,600 billion in 2020, accounting for 20% of the GDP. Besides, it was shown in the OECD report that in 2011, American people’s average expenditure on health care was up to 8233 dollars, twice the expenditure of comparatively developed European countries like French, Switzerland and England. In March, 2010, American president Barack Obama carried out the “Affordable Care Act”, aiming at improving the quality of American health insurance service, and decreasing the cost of health care service. The “ACA Act” established the balance sharing plan, which encouraged doctors and hospitals to meet the patients’ medical needs by an integral medical service system through encouraging accountable care organizations. Under that balance sharing plan, suppliers and organizers of the service who meet the standard set by US HHS can serve those who benefits from the medical insurance plan through co-organizing and coordinating. ACO could help reduce the cost and increase the efficiency, and distribute part of the surplus among members. Under the design of the ACO system, the new service supplying mode integrate hospitals, general practitioners, medical specialists and other specialists to answer for the medical quality and medical cost together.

In America, POCT is supervised by FDA, and is co-organized by certification authority, POCT committee, and POCT coordinators. It exists as one of the branches of IVD. Therefore, it is also supervised under the policies of medical apparatus and instruments. At the same time, IVD products are also supervised under CLIA 88 enacted in 1992, which divided IVD into three registering levels (low, medium and high) basing on the complexity of experiments. Before obtaining the right of market entrance, producers have to make appliances according to CLIA categorizing system. After that, CMS, DCLD and CDC will check the products and authorize corresponding CLIA certifications. When it comes to POCT products specially, they will have to firstly recognized by JCAHO and CAP, and finally authorize and certificate by JCAHO, CMS and CAP.

In terms of the usage of POCT products, owing to the fact that there is not much barrier, the superintendence is therefore the key to optimizing POCT and enlarging its range of application. POCT committees have been set in every organization, city and state in America so as to implement quality-superintendence over its usage and guarantee the accuracy of the testing results.
Working on synthetic biology in a lab is amazing, but sharing our enthusiasm can be even more wonderful. For the science education, we worked with people from all walks of life including teenagers, citizens, freshmen and XMU students.
This year our team XMU-China came to Xiamen Science and Technology Museum to advocate the synthetic biology and our project. The whole project about 3 parts, game, Poster and Wish Wall. First, the game part, children play the roles of antibody and antigen respectively, they battled to be the winner by RPS. After the game they all left a deep impression of the general method that our body fight with cancer. Second,Poster,we had some talkative teammates to take charge of this part, so many student from high school and primary school were attracted ,they crowded in front of the poster and listened to our announcers absorbedly. Finally, the Wish Wall part, after our diverse introductions about the synthetic biology, they had learn a lot about it, and some of them even inspire to give their contributions to synthetic biology, and want to attend the innovative, fantastic competition —— iGEM. It’s also a cheerful Day for our team to propagate the synthetic biology to so many children.



Fighting for Mooncake is a traditional activity spent with relatives or close friends in Xiamen area in order to celebrate the Mid-Autumn Festival. Throughout the game, we advertised the iGEM to freshmen, and introduced our project this year. It was worth mentioning that we also collected solicited opinions from all the students about the feasibility of our disease detective device and treatment device with cell-free system this year. 86.7% of participants showed positive attitudes towards our detective device, with the common sense of this device could performed pre-diagnosis quickly to guide patients. 66.8% students thought that it was difficult to treat tumors with vesicles, but it was very promising.




In order to popularize the common knowledge of biology to the public and meanwhile to draw the public attention to synthetic biology, this year we XMU-China team visited to Yanwu Elementary School, volunteering to teach elementary students biology, introducing our team and work, and directing them to do some simple biological experiments. For instance, we let them extract DNA from bananas.
In the course of our introduction, the children of Yanwu Primary school expressed their great enthusiasm, responded positively to our interaction, and moreover they showed terrific zest for our introduction. In these next simple experiments, the children were thrilled to be involved in one of the experiments.
In the end, through our teaching and guidance, most of the children have achieved remarkable results during the experiments. After the event, Yanwu Elementary school students said they learned a lot of useful knowledge, and very happy to do these interesting experiments by themselves within our guidance.
Also, the teacher fed back that they think this activity is so meaningful, extremely hoping to hold more such events in the primary school campus.


● Sequence of SYL3C-TEG-biotin:
5'-CACTACAGAGGTTGCGTCTGTCCCACGTTGTCATGGGGGGTTGGCCTG-TEG-Biotin-3'
● Sequence of C3-FITC:
5'-CTCTGTAGTGTTTTTTTTTTTTTT-FITC-3'
● Sequence of C4-FITC:
5'-TCTGTAGTGTTTTTTTTTTTTTTT-FITC-3'
● Binding Buffer (pH 7.3~7.5)contains:
1×PBS Buffer with 5 mM MgCl2
● SYL3C-TEG-biotin (BoruiTM, see Appendices)
● C3-FITC (BoruiTM, see Appendices)
● EpCAM (ACROBiosystems®)
● Magnetic Beads (InvitrogenTM)
● Binding & Washing Buffer (see Appendices)
● Incubation Buffer (see Appendices)
● Vortex (IKA® lab dancer)
● Fluorescence Spectrometer (Shimadzu® RF-6000)
1.8 μL of 100 μM SYL3C-TEG-biotin is diluted by 1×B&W Buffer up to 75 μL, and 1.2 μL of C3-FITC is also diluted by 1×B&W up to 50 μL.
The tube of 100 μg/mL C3-FITC should be coated properly by tin foil in order to avoid being quenched.
1. Make distributions in 1.5 mL centrifuge tubes according to the following:
Tube 1 (Blank Control): 100 μL incubation Buffer.
Tube 2 (Negative Control): 25 μL diluted SYL3C-TEG-biotin + 25 μL 1×B&W Buffer.
Tube 3 (Competition Group): 25 μL diluted SYL3C-TEG-biotin + 25 μL diluted C3-FITC.
Tube 4 (Competition Group without EpCAM): 25 μL diluted SYL3C-TEG-biotin + 25 μL diluted C3-FITC.
Tube 5 (Positive Control): 0.6 μL 100 μM C3-FITC + 100 μL incubation Buffer.
Note: The Tube 3, 4 and 5 should be coated by tin foil properly after distributing.
2. Denature, anneal and renature:
The Tube 2, 3, and 4 are denatured at 95°C for 5 minutes, then anneal to room temperature.
3. Wash magnetic beads:
1) Resuspend the beads and vortex for 10 seconds to make them even.
2) Transfer 75 μL of 10 mg / mL beads to 3 200 μL centrifuge tubes (ie 25 μL per tube).
3) Add 25 μL of 2×B&W Buffer to each tube.
4) Vortex for 5 seconds to resuspend.
5) Place the tubes in a magnetic field for 1 minute, then aspirate the supernatant.
6) Remove the tubes from the magnetic field and add 50 μL of 1×B&W Buffer to each tube.
7) Repeat steps 4) to 5).
8) Add 50 μL of 1×B&W Buffer to each tube.
4. Bind to magnetic beads:
1) Add 50 μL of solution of Tube 2,3 and 4 from step 2 to 50 μL of washed beads, respectively.
2) Vortex for 5 seconds to resuspend.
3) Incubate at room temperature for 30 minutes in the dark.
4) Vortex for 5 seconds to resuspend.
5) Place the tubes in a magnetic field for 2 minute, then aspirate the supernatant.
6) Remove the tubes from the magnetic field and add 100 μL of 1×B&W Buffer to each tube.
7) Repeat steps 4) to 5).
8) Add 100 μL of incubation Buffer to Tube2, 3 and 4, respectively.
5. Compete:
1) Add 17 μL of diluted EpCAM to Tube 2 and 3. And add 17 μL of incubation Buffer to the last each tube.
2) Incubate at room temperature for 40 minutes in the dark.
3) Place the tubes in a magnetic field for 2~3 minutes, then pipette 100 μL of the supernatant into a new 200 μL centrifuge tube and mark it.
4) Measure the fluorescence intensity of Tube 1, 5 and the supernatant from 3).
Note: Before measuring, the solution of each tube will be further diluted by adding 3, 000 μL of incubation Buffer into it. The excitation wave length is 495 nm, and the range of emission wave length is from 500 nm to 600 nm.
● Sequence of SYL3C-TEG-biotin:
5'-CACTACAGAGGTTGCGTCTGTCCCACGTTGTCATGGGGGGTTGGCCTG-TEG-Biotin-3'
● Sequence of C3-FITC:
5'-CTCTGTAGTGTTTTTTTTTTTTTT-FITC-3'
● 2×Binding & Washing Buffer (pH 7.5) contains:
10 mM Tris 1 mM EDTA 2 M NaCl
● Incubation Buffer (pH 7.5) contains:
20 mM Tris 140 mM NaCl 5 mM KCl 1 mM MgCl2
● Cas12a (Alt-R® CRISPR-Cas12a (Cpf1) System)
● crRNA (IDT®)
● TE Buffer (Sangon Biotech®)
● DNaseAlertTM Substrate Nuclease Detection System (IDT®, see Appendices)
● 21nt ssDNA (BoruiTM, see Appendices)
● Black 96-well plate (Corning®)
● Microplate reader (Tecan Infinite® M200 Pro)
● Add 26.7 μL of filtered TE Buffer into the tube of crRNA to make a 75 μM stock.
● Add 88.6 μL of filtered TE Buffer into the tube of ssDNA to make a 100 μM stock.
1. Form the RNP complex:
1) Prepare 3 200 μL tubes. Add 1 μL of Cas12a and 1 μL of diluted crRNA into each nuclease-free 200 μL tube.
2) Incubate Cas12a with crRNA at 37℃ for 30 minutes to form the RNP complex.
2. Pretreatment of substrate:
1) Prepare 3 DNaseAlertTM Substrate single-use tubes. Add 5 μL of Nuclease-Free Water to each tube.
2) Add 5 μL of 10×DNaseAlertTM Buffer to each tube.
3. Make distributions:
1) Cas12a Group: DNaseAlertTM Substrate (from step 2) + 2 μL RNP complex (from step 1) + 1 μL diluted ssDNA + 37 μL filtered TE Buffer + 40 μL Nuclease-Free Water.
2) Negative Control: DNaseAlertTM Substrate (from step 2) + 40 μL Nuclease-Free Water + 40 μL Nuclease-Free Water.
3) Positive Control: DNaseAlertTM Substrate (from step 2) + 1 μL DNase I + 39 μL filtered TE Buffer + 40 μL Nuclease-Free Water.
4. Measure fluorescence intensity by microplate reader:
1) Incubate the reaction system at 37℃ for 30 minutes, then transform the reaction system into the black 96-well plate.
2) Before measure, add 12 μL of TE Buffer into each tube.
3) Set temperature: 37℃, choose plate type: Black. Set excitation wavelength: 536 nm and emission wavelength: 556 nm. Choose mode: Optimal.
4) Measure fluorescence intensity at 30 min, 40 min, 50 min, 60 min, 70 min, 90 min, 110 min (Timing is from the beginning of incubation).
● DNaseAlertTM Substrate Nuclease Detection System contains:
1) DNaseAlertTM Substrate (25 single-use tubes (50 pmol per tube) or 2 tubes bulk substrate (2 nmol per tube))
2) DNaseAlertTM Buffer (250 μL)
3) Nuclease-Free Water (2 mL)
4) DNase I Enzyme (25 μL)
5) Nuclease Decontamination Solution (50 mL)
● Sequence of 21nt ssDNA:
5'-CAGGCCAACCCCCCATGACAA-3'
● TE Buffer (Sangon Biotech®)
● DNaseAlertTM Substrate Nuclease Detection System (IDT®, see Appendices)
● Fluorescence spectrometer (Shimadzu® RF-6000)
1. Make distributions:
Add reagent into 2 DNaseAlertTM Substrate single-use tubes according to following instruction. Avoiding light is necessary during the whole process.
1) Negative Control: 5 μL Nuclease-Free Water + 5 μL 10×DNaseAlert Buffer + 40 μL Nuclease-Free Water + 40 μL Nuclease-Free Water.
2) Enzyme Group: 5 μL Nuclease-Free Water + 5 μL 10×DNaseAlert Buffer + 39 μL Nuclease-Free Water + 40 μL Nuclease-Free Water.
Place the two tubes at room temperature for 30 minutes to enough dissolve fluorescent substance.
2. Make distributions further according to the time gradient:
1) Prepare 6 200μL-PCR tubes in advance, then divide into 2 groups (3 tubes per group). Mark "W" and "I" on the tubes of two groups, respectively. Further mark the tubes according to the time gradient of 3 min, 15 min and 30 min (e.g. "W 3", "I 15").
2) Add 1 μL of DNase I into the DNaseAlertTM Substrate single-use tube of Enzyme Group.
3) Mix immediately, then transfer 30 μL of reaction liquid from each single-use tube to each PCR tube marked before. Place all PCR tubes in 37℃ water bath. Then start timing.
4) Each tube: React for corresponding time, then measure florescence intensity immediately.
3. Measure fluorescence intensity:
1) Turn on the instrument for 30 minutes before measuring.
2) Choose "Emission Mode". The excitation wave length is 536 nm, and the range of emission wave length is from 540 nm to 580 nm.
3) Before measuring, dilute the reaction liquid to about 3 mL with TE Buffer in the fluorescence cuvette.
● DNaseAlertTM Substrate Nuclease Detection System contains:
1) DNaseAlertTM Substrate (25 single-use tubes (50 pmol per tube) or 2 tubes bulk substrate (2 nmol per tube))
2) DNaseAlertTM Buffer (250 μL)
3) Nuclease-Free Water (2 mL)
4) DNase I Enzyme (25 μL)
5) Nuclease Decontamination Solution (50 mL)

