Demonstration
This year we desired to create a light-induced metabolic flux redirection platform in engineered bacteria. To achieve this idea, we improved the light sensor CcaS/CcaR system. In addition, we measured the type I-E CRSPRi system and combined it with advanced photoreceptor to control the metabolic flux dynamically.
Now we are proud to present our results:
(1) We successfully created three new types of CcaS/CcaR system: #3, #4, #10.
(2) We constructed a metabolic flux redirection platform dynamically controlled by light.
(3) We demonstrated our system through the production of Polyhydroxybutyrate(PHB).
(4) We manufactured illuminated hardware through reconstructing the shakers suitable for further fermentation.
We removed two PAS domains in CcaS and acquired #3、#4、#10 variants of CcaS. We tested these three variants with the wild type and measured their expressions of sfGFP in the dark state or under light (green or red) state respectively. The measurements showed that #3 and #10 have similar response to green/red light and are better than the original one.

To demonstrate our dynamic light-activated redirection system, we tried to change the induction conditions at different stages of growth. The samples were illuminated by red light at the beginning, then induced with green light at early-log phase (2h), mid-log phase(5h) and late-log phase(9h). As you can see in the figure that among all conditions, bacteria growth was repressed the most obviously when induced at mid-log phase (5h), whereas induction at late-log phase (9h) had little impact on bacteria growth. Through this dynamic platform, we can arbitrarily regulate the expression of targeted gene at different stages to control the metabolism in bacteria to optimize the production of biological products.

n addition, we analyzed the metabolic flux before and after the shift through the FBA model, and the computational result also certifies that original metabolic flux was affected by targeting gltA gene as well as TCA Cycle
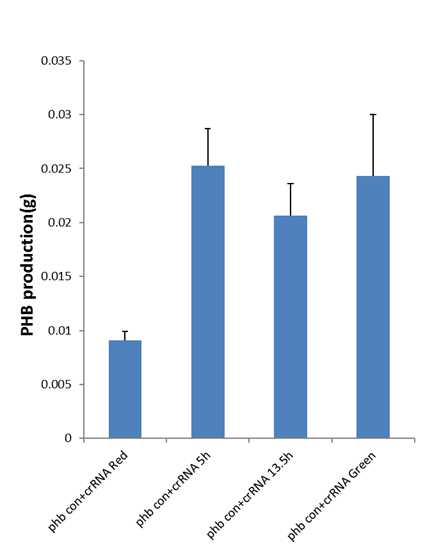
To introduce this system into metabolic engineering and demonstrate that it would make sense in production, we took the fermentation of producing PHB as an example. We constitutively expressed phbCAB cluster and induced the expression of crRNA through different light. An obvious variance in PHB production(g) and PHB content (%) between red and green light had shown in results. What’s more, the data indicated that the expressing crRNA induced at different phases of fermentation led to the difference of PHB content (%) and production(g), in which promoting by switching to green light at 5h (exponential phase) had significant increase in PHB fermentation. It demonstrated that our light-induced metabolic flux redirection platform worked well in metabolic engineering.

For the convenience of the experiments and further fermentation, we also reconstructed the shakers for erlenmeyers in which the volume ranging from 50ml to 1000ml. This equipment can change the light color, light intensity, even the irradiation frequency. Such a multi-functional ameliorated hardware provides a solid foundation for further study in light.


Future
1) Since we have confirmed that PHB production and type I-E CRISPRi system could work well respectively, so the next step we need to do is combining these two systems together to accomplish the final switch and test the whole metabolic platform by measuring bacteria growth curve and production of PHB.
2) optimize the function and performance of light sensor and make it more suitable for metabolic engineering and industrial fermentation, especially the sensitivity since the non-transparent culture medium is a unignorable burden.
3) In order to decrease the leakage of light-induced CRISPRi system, a further study of compatibility between light sensor and type I-E CRISPRi system must be carried out.
4) Analysis of metabolic flux through HPLC or isotope labeling for the change of metabolic flow to ensure that metabolic flux did be redirected induced by light.
5) PHB might not be a best choice to demonstrate the system. Choose another product as acetic acid which comes from acetyl-CoA directly in case of the competition of other pathways.
6) Due to the high orthogonality of the light sensing systems, it is valuable to introduce multiple light sensing system into metabolic engineering as well as industrial fermentation. The next step of our process is to introduce another light sensing system into our project controlling another synthesizing pathway.


