Contents
Background
In this year's project, we focused on the biological production of the building blocks, the so-called monomers, of the polymers PLGA (Poly(lactic-co-glycolic-acid)) and PLGC (Poly(lacticlactice-co-glycolide-co-ε-caprolactone)). The needed monomers for these polymers are lactic acid, glycolic acid, and ε-caprolactone. Since some former iGEM Teams, e.g. the iGEM-team from Evry in 2016, already worked with lactic acid, we focused on producing ε-caprolactone and glycolic acid. Another reason why we did not focus on lactic acid is, that it is already industrially produced in large scale by bacterial fermentation of sugars obtained from renewable resources, making it a largely eco-friendly product. [1]
The problem we want to tackle
For the production of these two compounds, currently a lot of harmful chemicals are needed. Glycolic acid can be produced in two different manners. Either it is produced by an alkaline hydrolysis of chloric acid, or it is synthesized out of water, carbon monoxide and formaldehyde. [2]
Formaldehyde and chloric acid are both toxic for humans and may harm exposed persons long-term. Furthermore, most of the chemicals needed for the production of glycolic acid are obtained from petrochemicals. Petrochemicals are derived from petroleum or other fossil fuel, e.g. natural gas and coal. The resources for these are finite, and it is therefore concerning that society is depending on them. Additionally, for the retrieval of them, nature often has to be destroyed, and during the retrieval, gases and aerosol are released, which may cause cancer. Another problem, especially with fossil oil, is that it is often drilled illegally, which increases the negative impact on the environment by e.g. destroying soil. ε-caprolactone is produced in a multi-10,000 ton scale per year from cyclohexanol, acetic acid and hydrogen peroxide. ). [3] Acetic acid is mostly also derived from petrochemicals. Hydrogen peroxide and cyclohexanone are also toxic for humans, and hydrogen peroxide is environmentally harmful, as well. Furthermore, hydrogen peroxide has only a modest selectivity (80-90%) which leads to a loss of product.
To overcome the above mentioned problems, and to establish an environmentally friendly and safe way to produce the named monomers with high yields, is the goal of this year's iGEM TU Darmstadt project. In order to be able to compete with chemical production, we need to achieve high production yields. We chose Escherichia coli and Saccharomyces cerevisiae as host organisms, to allow a comparison and to draw conclusions, which one is better suited for large scale production of our desired monomers.
You might wonder what PLGA and PLGC are
Like many other common plastics, e.g. Polycarbonate (PC) or Polyethylenterephthalate (PET), Poly(lactic-co-glycolic-acid) belongs to the family of polyesters. PLGA is built from lactic acid and glycolic acid monomers, and is characterized by both a high mechanical resistance and being a thermoplastic. The monomers lactic acid and glycolic acid are both bi-functional alpha-hydroxy carboxylic acids without any further reactive groups. This leads to a linear combination of the monomers without cross-links. Cross-links are covalent bonds that interconnect separate linear polymer chains and thereby give rise to a polymer-network. Polymers without such cross-links, such as PLGA, are called thermoplastics.
There are two more types of polymers which are characterized through their degree of cross-links. Polymers with a small degree of cross-links and certain distances between them, leading to a flexible and formable structure at standard ambient conditions, are called elastomers. Polymers with a large degree of cross-links, leading to a really hard and mechanical resistant structure even at high temperatures, are called thermosetting plastics. Depending on the application, it is necessary to choose between these three possible polymer types.
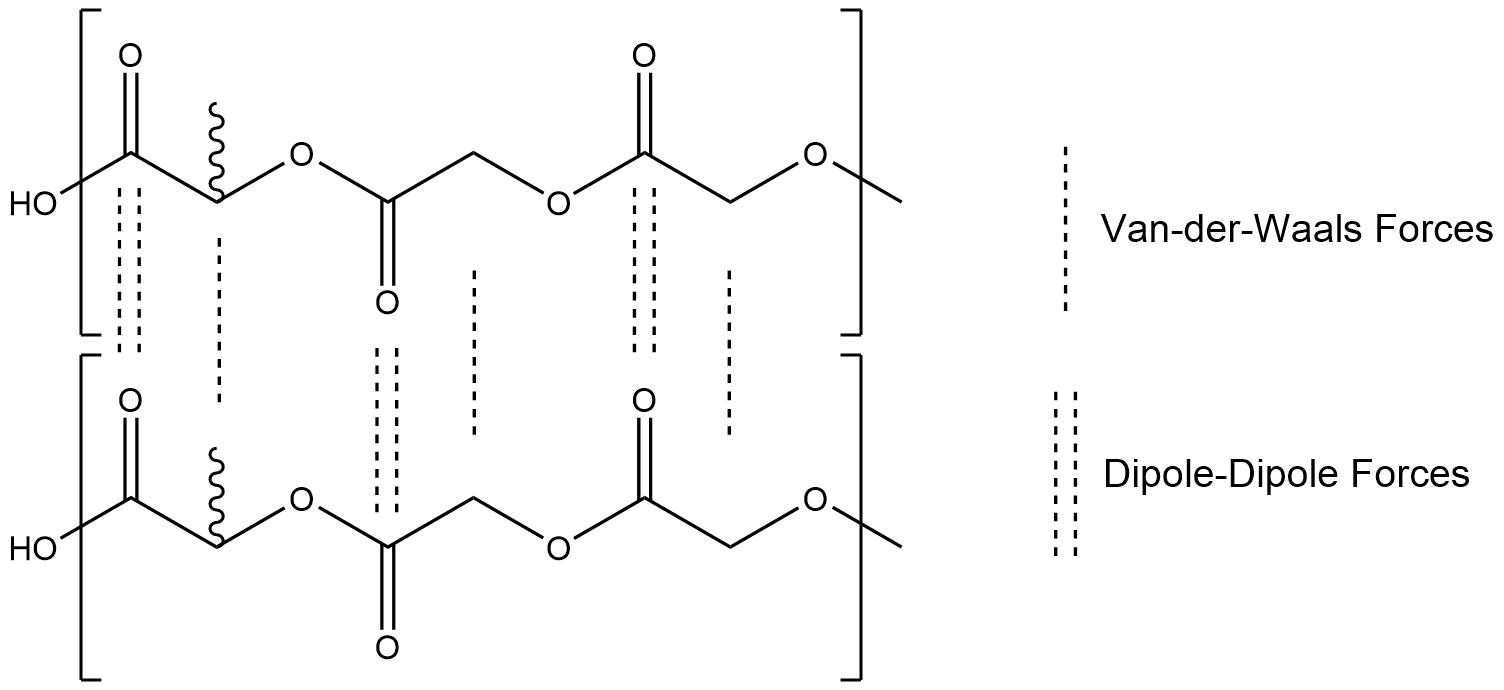
In a thermoplastic, Van-der-Waals-Forces and slightly stronger dipole-dipole-interactions are acting between the polymer chains. Given that our monomers only consist of chains of small monomers, the amount of dipole-dipole-interactions is high, which leads to strong intermolecular forces. Under standard ambient conditions, PLGA is a hard solid with high mechanical resistance. Through heat it is possible to overcome these intermolecular forces, which leads to an increase of flexibility, up to the melting of the polymer. The melting process is called glass transition range. In a thermoplastic with stronger intermolecular forces, the glass transitions range begins later and the required temperature is higher. There are two more types of polymers which are characterized through their degree of cross-links. Polymers with a small degree of cross-links and certain distances between them, leading to a flexible and formable structure at standard ambient conditions, are called elastomers. Polymers with a large degree of cross-links, leading to a really hard and mechanical resistant structure, even at high temperatures, are called thermosetting plastics. Depending on the application, it is necessary to choose between these three possible polymer types. [4]
Figure 1: Chemical Interactions between PLGA polymer chains
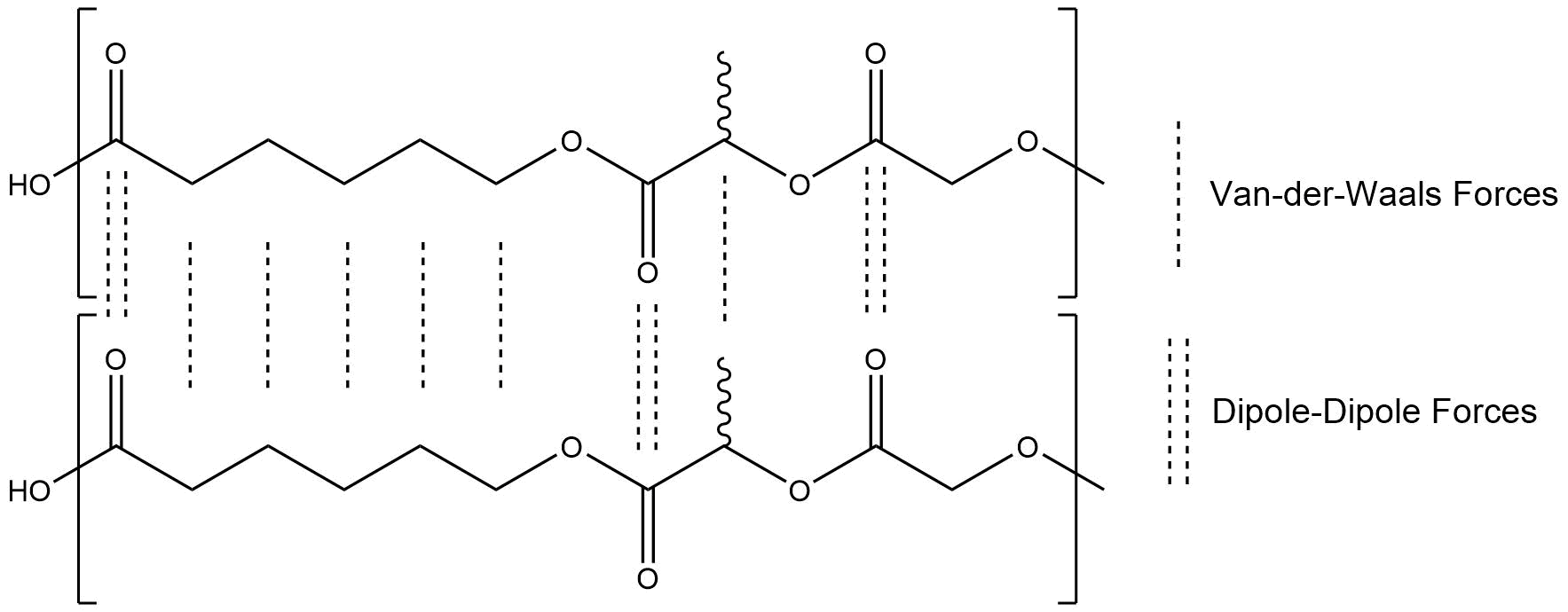
Like PLGA, PLGC is a thermoplastic polyester. The structural difference is, that PLGC additionally includes ε-caprolactone. ε-caprolactone also has only two reactive groups, PLGC is therefore a thermoplastic like PLGA. Nevertheless, both polymers have differences in their properties. PLGA has a higher mechanical resistance because ε-caprolactone has six linear carbon atoms in it. The frequency of the ester groups, and therefore the amount of dipole-dipole interactions, is lower. As a consequence, PLGC also displays a lower glass transition range.
Figure 2: Chemical Interactions between PLGC polymer chains
Properties of our polymers in a biological system
Both of our polymers are biodegradable and biocompatible. A biodegradable material can degrade in a natural environment into smaller compounds, which are not harmful. Eventually, it degrades into simple compounds like oxygen, carbon dioxide and water. Biodegradation is catalyzed by either organisms like bacteria and fungi, or by other natural factors like water, UV and oxygen. Biodegradable plastics refer to a plastic which is strong during the period of use, but after a defined time-frame degrades into compounds which are non-toxic.
[5]
Since PLGA and PLGC meet all these criterias, they are considered biodegradable.
[6] [7]
A biocompatible polymer refers to a biomaterial which is compatible with living tissue, and is therefore able to substitute a part of a living systems’ function in contact with it. It is furthermore a material which does not harm the body, and does not create inflammatory reactions. Biocompatible polymers are designed to interface, and depending on the composition, to interact with biologically active surroundings. Because of that, they are important for clinical applications such as implants, drug delivery systems, and tissue engineering, and are often used in modern medicine.
[8]
Both PLGA and PLGC are also biocompatible polymers.
[9]
When PLGA and PLGC are degrading, they undergo surface or bulk erosion, depending on e.g. their size, weight and density. Surface erosion means, that the polymer degrades from the outside to the inside, while during bulk erosion, water diffuses through it and it degrades at almost the same rate in every place of the polymer. PLGA and PLGC degrade into acids, which lower the pH and thereby catalyzes the degradation process. This process is called autohydrolysis. The process has also an impact on implants and nanospheres in humans, even though the blood maintains a constant pH value. When the polymer reaches a critical size, diffusion dominates and bulk erosion takes place. The polymer degrades into the acids, leading to an accumulation of acid inside the polymer structure, since they are not able to diffuse out of the polymer again. This results in a pH-gradient from the inside to the outside of the nanosphere or implant, which in turn leads to a faster degradation on the inside where the pH is lower. When degradation advances, this leads to pore formation. Due to this fact, their degradation is not linear. [11]
Why would we be interested in PLGA and PLGC?
You might already know about PLA (poly-lactic-acid), which is an established biodegradable polymer and has a long life time. It is used e.g. for packaging materials and disposable cups. (see figure XX, CHART) PLGA is made out of lactic acid and additionally glycolic acid. Glycolic acid speeds up the degradation process, and with the ratio of glycolic acid to lactic acid the degradation time can be varied. The ε-caprolactones, which is needed additionally for PLGC, further modifies the degradation behavior of the polymer by making the ester bonds easier accessible for water, which speeds up the degradation process. The amount of ε-caprolactone can therefore influence how fast or slow the polymer is degraded.
[12]
This makes PLGA and PLGC suitable for medical applications, such as implants, tissue engineering and drug delivery systems, where the polymer can degrade in a predetermined timeframe. Depending on the application, this can be anywhere from weeks to months. PLGA degrades into its building blocks in the body. As they can be metabolized, it is approved by the [7]FDA for clinical usage.
[13]
ε-caprolactone cannot be metabolized, but gets excreted by the human body without doing any harm. [14]
Another polymer which can be produced with ε-caprolactone is PCL (poly-ε-caprolactone). It has a longer lifetime than PLGC, due to minimal acid generation while degrading, which in turn does not lead to a strong autocatalysis. This makes this polymer suitable for long-term medication via nanospheres, as well as implants. [15] With the monomers we aim to produce, the formation of PCL would also be possible.
Comparison of our polymers with other polymers
Another problem we are facing today, is the amount of microplastics found in our environment, especially in our oceans. Microplastics are plastic particles with a diameter of less than 5 mm, which are released by e.g. plastic trash and cosmetic products. Microplastics enter the nutrition chain via water organisms, which in the end can end up in the human body. Furthermore, a lot of plastics incorporate substances like softening agents, which can potentially cause cancer. Since our polymer degrades in water into metabolizable monomers, it does not cause these problems, and is therefore an ecofriendly option.- ↑ Komesu, A., Oliveira, BioRes. 12(2). 4364-4383, 2017
- ↑ patents.google [1]
- ↑ Sandy Schmidt, Angewandte Chemie, Wiley Online Library, 2015.
- ↑ classes of polymers [2].
- ↑ Ikada Y, Tsuji H Macromolecular Rapid Communications. 21 (3),2000
- ↑ Ji Hoon Park, Journal of Materials Chemistry B,2015
- ↑ 7.0 7.1 Qing Cai, Polymers for advanced technologies 13, 105-11, 2002
- ↑ Ernest W.Lau, Elsevier, 2017.
- ↑ Hirenkumar K. Makadia, Polymers 2011
- ↑ Diederik J. Opperman,The Royal Society of Chemistry 2015, 2015.
- ↑ Friederike von Burkersroda, Biomaterials 23 (2002) 4221–4231, 2002
- ↑ Ji Hoon Park, Royal Society of chemistry,2015
- ↑ FDA decision about PLGA https://www.millioninsights.com/industry-reports/poly-lactic-co-glycolic-acid-plga-market?utm_source=pressrelease&utm_medium=referral&utm_campaign=Abnewswire_Shweta_Sept25&utm_content=Content.
- ↑ Yodthong Baimark, ScienceAsia 30, 2004
- ↑ Adam L. Sisson, Elsevier, 2013