Contents
Proof of Concept
Summary of our Achievements
Successful in vitro and in in vivo production of glycolic acid in E. coli.
Successful in vitro production of glycolic acid by metabolic
modified Saccharomyces cerevisiae.
Successful in vitro production of ε‑caprolactone in E. coli.
Successful production of PLGA and PLGC.
Production of nanospheres loaded with fluorescein as one possible
application of PLGA.
Development of a kinetic model of our polymerization.
Integration of the feedback from various scientists and the public
into our project.
We managed to produce our monomers, and thereby also our
polymers, in a sustainable and environment-friendly way.
Glycolic Acid Production in E. coli
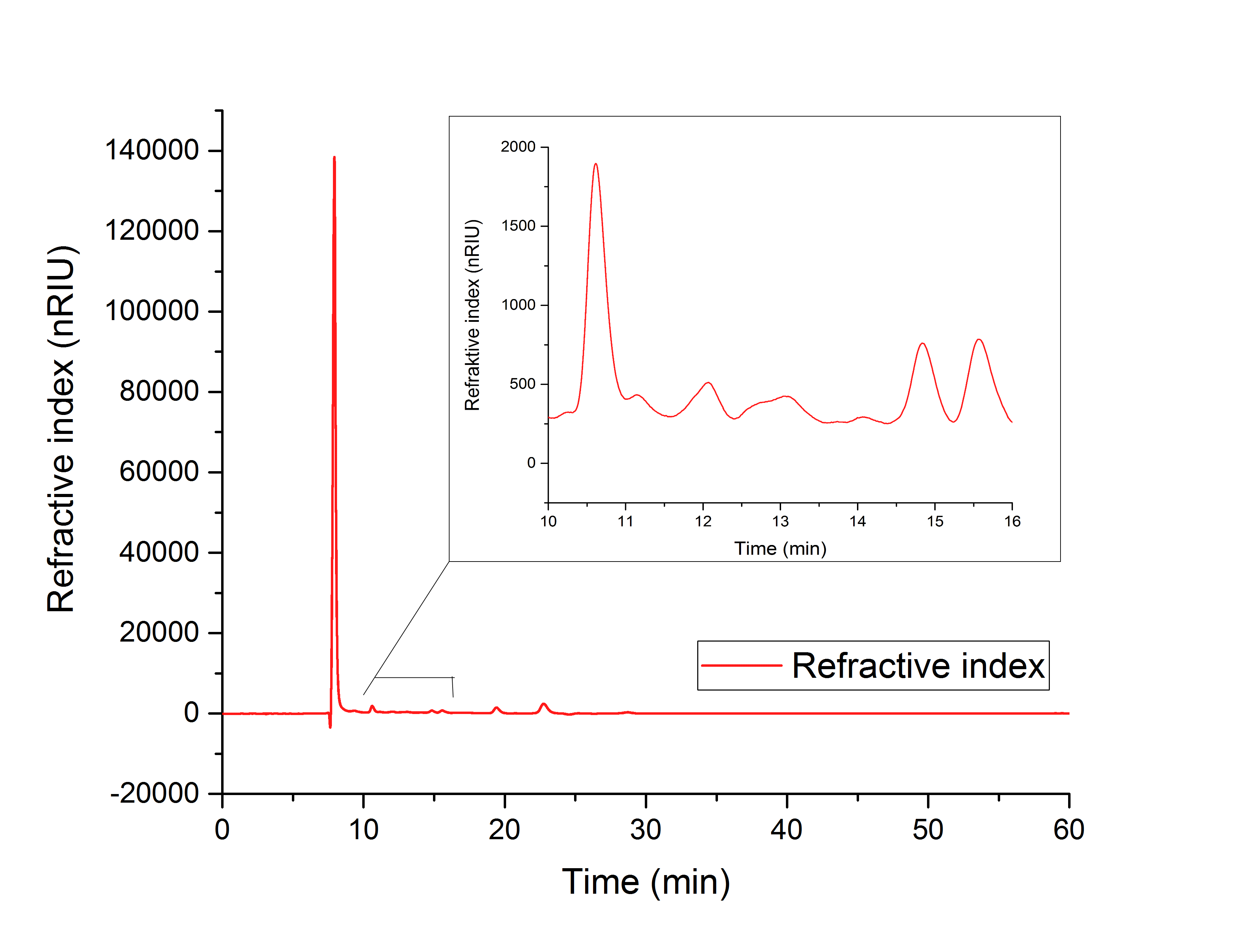
Our aim was to produce glycolic acid for the production of biodegradable polymers. We were able to overexpress the genes aceA and ycdW in Escherichia coli. The proteins AceA and YcdW were His-tagged and purified via an ÄKTA system. The purification was confirmed by an SDS-PAGE and a western blot. After that, we successfully characterized YcdW with an NADPH dependent enzyme assay. The assay indicated, that YcdW uses glyoxylate as substrate under consumption of NADPH to produce glycolic acid. The functionality of AceA was verified with a phenylhydrazine-dependent assay. AceA catalyzes the reaction from isocitrate to glyoxylate. The enzyme assays were performed a second time over a longer incubation period and analyzed via HPLC. The expected products of the reactions were found, indicating a catalytic activity of the purified enzymes. When the enzyme YcdW was produced in E. coli, the formation of glycolic acid was observed in the disrupted cells (Figure 1). The combined evidence shows that the transformation of E. coli was successful, leading to the production of functional enzymes. These enzymes then partake in the production pathway of glycolic acid, which was shown in vivo, as well as in vitro. Thus, we could successfully show the production of the first monomer for our biopolymer.
Figure 1: HPLC analysis of supernatant of disrupted ycdW transformed E. coli. Glyoxylate and glycolic acid peaks can be seen at 12.066 and 15.568 minutes.
For more detailed information please look here.
Glycolic Acid Production in S. cerevisiae
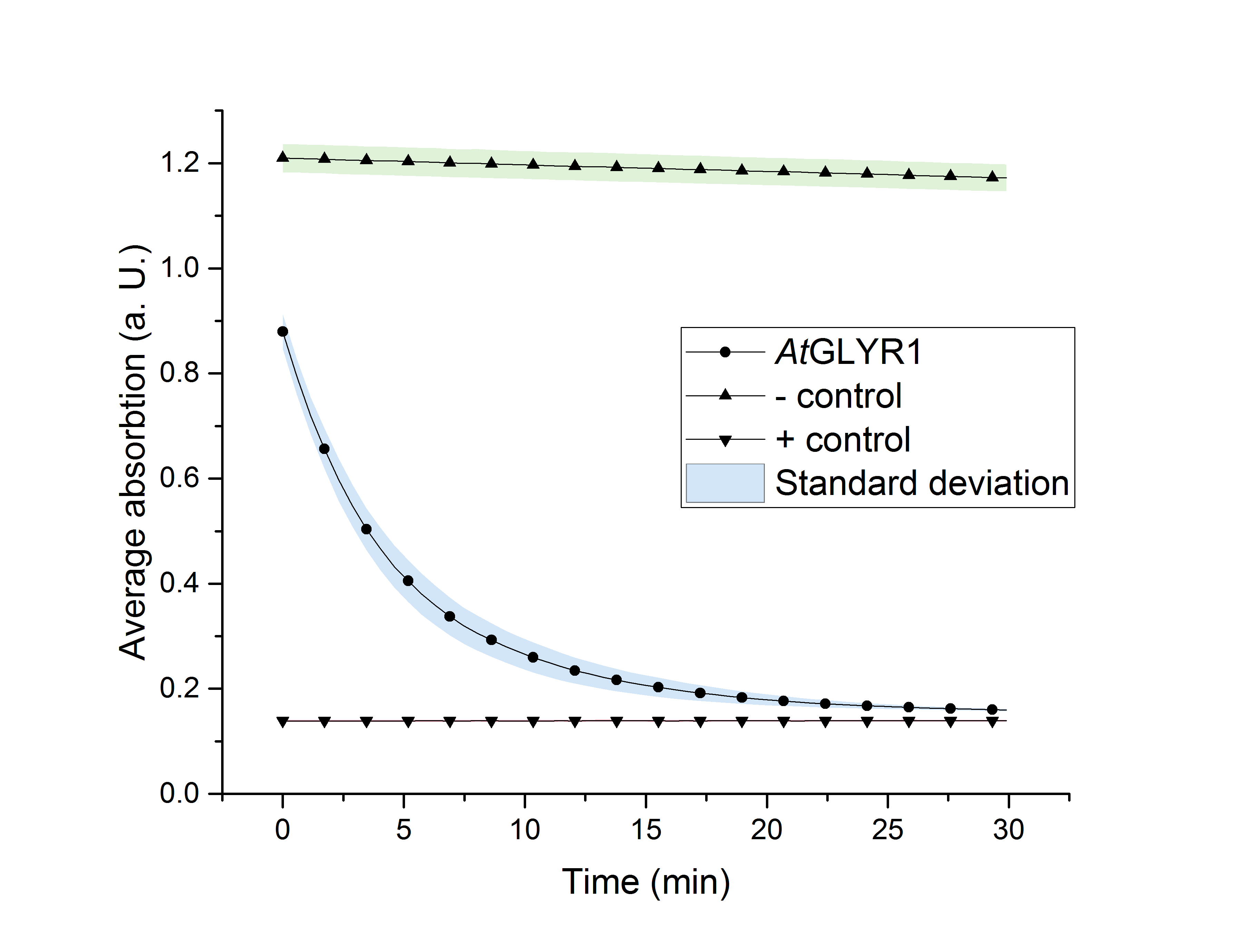
As glycolic acid is one monomer which is needed to produce PLGA, our goal was to produce glycolic acid in Saccharomyces cerevisiae. We were successful in the heterologous production of AtGLYR1 in S. cerevisiae. This was shown by western blot analysis, as well as during an enzyme assay. Using a NADPH dependent enzyme assay, we could proof the functionality of our purified enzyme (Figure 2). During the assay, AtGLYR1 converts glyoxylate to glycolic acid, while turning over NADPH. Therefore, we were able to produce glycolic acid in vitro. Furthermore, we were able to delete several genes from the genome of CEN.PK-1C. This could be helpful to increase the production of glycolic acid in vivo.
Figure 2: NADPH dependent enzyme assay of AtGLYR1. The graph shows the average absorption at 340 nm in correlation to the time (min). For the enzyme assay 2.7 ng/µL was used, positive (NADP+) and negative control (NADPH) were included.
For more detailed information pease look here.
ε-caprolactone Production in E. coli
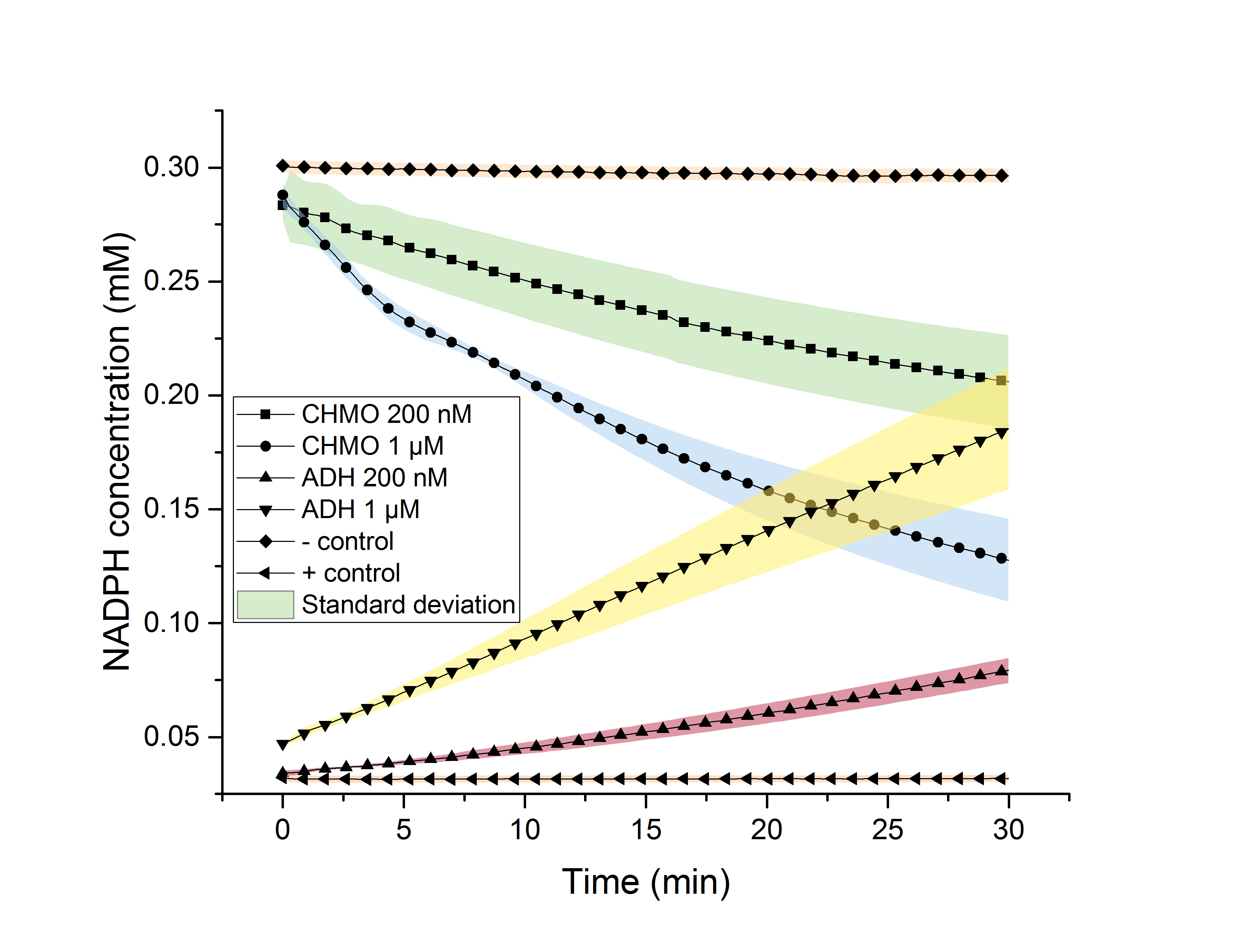
The aim of our project was to produce biopolymers, with ε-caprolactone as one potential monomer. To generate this monomer, we successfully overexpressed the genes for heterologous enzymes ADH and CHMO in E. coli. We were able to purify these enzymes using a His-tag. The purified enzymes were detected via SDS-PAGE and western blot. ADH produces NADPH while converting cyclohexanol to cyclohexanone, while CHMO uses NADPH, converting cyclohexanone to ε-caprolactone. An NADPH dependent assay was used to determine the activity of the enzymes separately, as well as in a combined assay (Figure 3 and 4).
Figure 3 (left): NADPH dependent enzyme assay of CHMO and ADH.
Figure 4 (right): NADPH dependent enzyme assay of CHMO and ADH in one reaction.
Enzyme activity could be shown in both cases.
The equilibrium of the production and depletion of NADPH in the combined assay indicates the synthesis of ε‑caprolactone, and thus the successful creation of the second monomer for our biopolymer.
For more detailed information pease look here.
Manufacturing of Polymers
We wanted to develop a protocol for the manual production of polymers using the monomers glycolic acid, lactic acid and ε-caprolactone. We were able to manufacture the polymers PLGA and PLGC out of their respective monomers, which we could show by gel permeation chromatography (GPC) analysis. We were also able to manually produce nanospheres out of these polymers, thereby showing the feasibility of our potential application scenario.
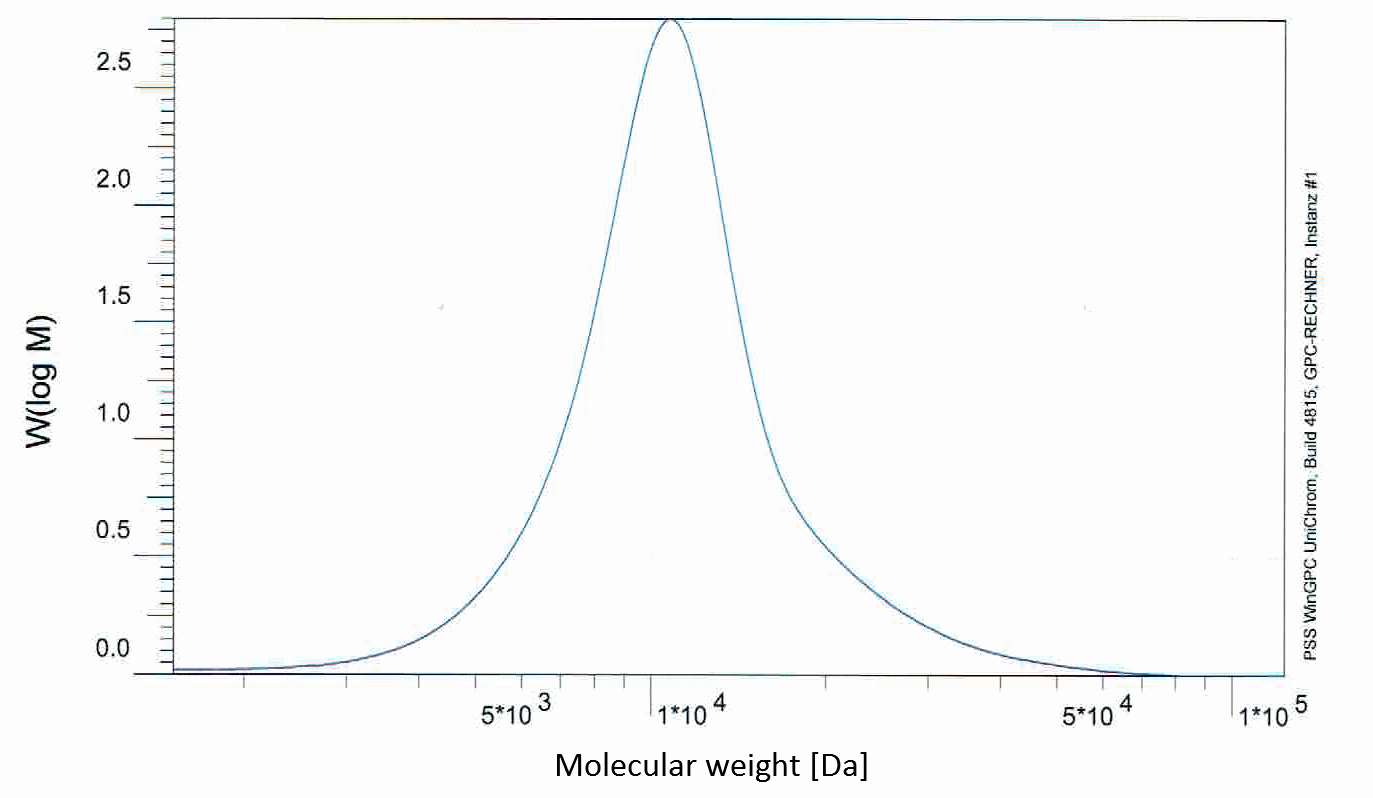
The GPC of PLGC shows a narrow distribution of a molecule weight of about 12,000 g/mol, as seen in figure 5.
Figure 5: Gel permeation chromatography of PLGC (I)
As its monomers show a molecular weight of 116 g/mol and 144 g/mol, polymer formation can be inferred.
For more detailed information pease look here.