Contents
Monomers
Abstract
An important step in the polymer industry is the production of the needed monomers. Their characteristics decide the properties of the final polymer. By modifying the metabolism of microorganisms, an environmentally friendly and sustainable way to produce glycolic acid, caprolactones and lactic acid can be established. These monomers are the basic components of the biodegradable polymers PLGA and PLGC.
Glycolic Acid
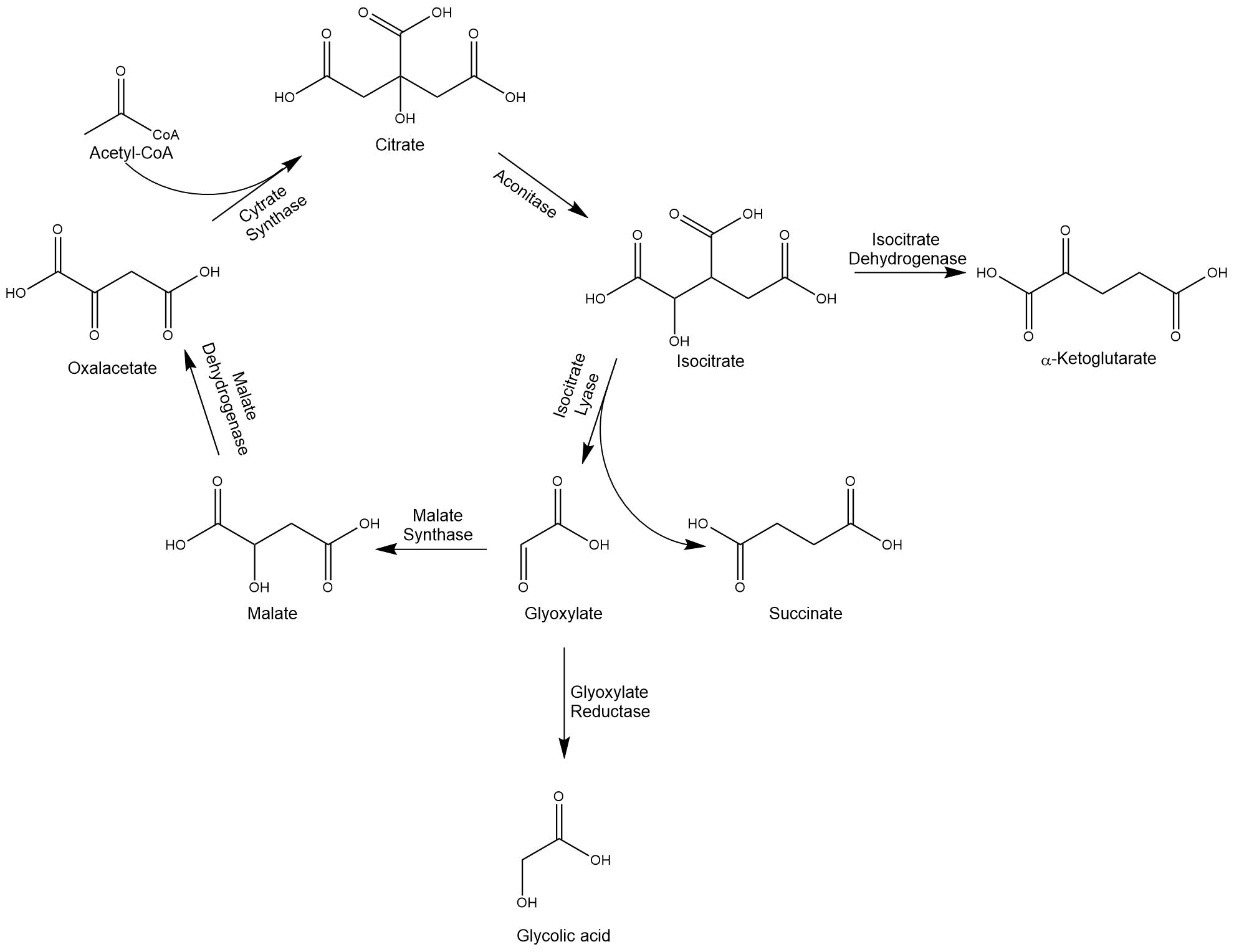
Glycolic acid is a simple α-hydroxy acid. Through the functional hydroxy- and acid-groups the molecule is highly soluble in water. This property makes glycolic acid polymers attractive for many applications in industry, e.g. in the textile, leather, oil, and gas industry[1]. The polymer of glycolic acid exhibits excellent gas barrier properties, which is an optimal base for e.g. packaging materials[2]. To enable the production of glycolic acid, we modified the glyoxylate cycle, which branches off the TCA cycle.
When acetyl-CoA yielding C2-units, e.g. acetate or ethanol, act as a sole carbon source for organisms such as E. coli and S. cerevisiae, the glyoxylate cycle functions as an anaplerotic pathway replenishing succinate.
The isocitrate lyase converts isocitrate into succinate and glyoxylate and thereby enables the bypassing of both decarboxylation steps of the TCA cycle. Another key enzyme is the malate synthase, which condenses glyoxylate with two acetyl-CoA molecules to isocitrate. The intermediate glyoxylate can also be reduced by a glyoxylate reductase to glycolic acid[3]. Because of the different characteristics of microorganisms, we decided to work with E. coli, as well as S. cerevisiae.
Figure 1: Modified glyoxylate cycle for the production of glycolic acid.
For more detailed information about the glycolic acid production in E. coli please look here. For the production in S. cerevisiae look here.
Caprolactone
ε-caprolactone is a seven-membered circular lactone. It is used as a building block for several polymers, like in our case poly(lactide-co-glycolide-co-caprolactone), abbreviated PLGC. The addition of ε-caprolactone to the polymer mix decreases its degradation time by making ester bonds easier accessible for water molecules. Therefore, polymers with caprolactones have a shorter lifetime than polymers without them. Right now, ε-caprolactone is derived from petrochemicals and further chemicals, which are harmful for living beings and their environment. In order to produce ε-caprolactone in E. coli, we used two enzymes, cyclohexanone monooxygenase (CHMO) and alcohol dehydrogenase (ADH).
For more detailed information about the ε-caprolactone in E. coli please look here.
Lactic Acid
In our project, we manufactured two co-polymers of polylactic-acid (PLA). For this, we genetically modified microorganisms to produce the monomers glycolic acid and ε-caprolactone. The monomer with the biggest mole fraction in these blends is lactic acid, whose synthesis requires acetaldehyde, obtained from petrochemicials, and the extremely toxic hydrogen cyanide. Another less toxic possibility to obtain lactic acid is through microorganisms. We did not produce this monomer ourselves, as iGEM Evry 2016 already tried this approach in Pseudomonas putida. To achieve this, they engineered P. putida's metabolic pathway. The parts they used, and further details, are shown on their page[4]. Poly-lactid-acid (PLA) is already a well-known biodegradable polymer and is used in several real-world applications, such as wrapping materials or suture stitches. Furthermore, a wide spread industrial production through microorganisms has already been established. Thus, we felt it was not necessary to include lactic acid production into our project.
Based on the results, of iGEM team Evry, we wanted to extend their idea of producing monomers biologically. We want to focus on the production of two other monomers that can be used for biological polymer production: glycolic acid and ε-caprolactone. With these three components, we aim to produce biodegradable co-polymers. The combination of biologically produced monomers and a biodegradable co-polymer brings the production of green polymers to the next level.
Model Organisms
Abstract
In our project we produced glycolic acid and caprolactones in E. coli and in S. cerevisiae. Both are common model organisms used in many laboratories and can be grown in relatively simple culture media. Their broad application is mainly attributed to their relative ease of genetic manipulation, allowing the production of heterologous proteins.
Escherichia coli
E. coli is probably the most used bacterium in biotechnology. It contains a stable heterologous expression system and fast growth rates. Because this system has been used for genetic manipulation since 1973, it is well characterized. In addition to fast growth rates, e.g. compared to mammalian cells, it offers the ability to produce proteins in large quantities. Nowadays, it is widely utilized in the production of pharmaceuticals, enzymes, biofuels and fine chemicals. It has to be noted, that the production of high amounts of protein can lead to the formation of insoluble inclusion bodies which often have to be refolded before further use.
Saccharomyces cerevisiae
S. cerevisiae is a routinely used organism for the industrial production of fuels and chemicals, e.g. lactic acid, and for the production of consumables like bread and beer[5]. Genetic engineerings benefit from its ability to be genetically modified via homologous recombination, allowing the efficient introduction of constructs into the genome and gene knock-out or knock-downs. Furthermore, the ability to secrete folded protein attached to signal sequences, as well asits high tolerance towards low pH values make S. cerevisiae a suitable organism for the biotech industry. The recognized safe status[6] simplifies the approval of processes and products and reduces the problem of waste disposal.
Dimer Synthesis
Abstract
To biotechnologically produce the monomers for the synthesis of PLGA and PLGC polymers, we genetically engineered the metabolism of Escherichia coli and Saccharomyces cerevisiae and thereby inserted an operon enabling the biosynthesis of glycolic acid in both host organisms. Furthermore, previous iGEM projects, e.g. Evry 2016, worked on the biosynthesis of lactic acid, e.g. in Pseudomonas putida. However, to achieve larger molecular weights of the PLGA and PLGC polymers, the utilization of the respective dimers of glycolic and lactic acid, glycolide and lactide, is beneficial. [7] To our knowledge the enzymatic conversion of the two monomers to their respective dimers is unknown, but the chemical synthesis is feasible. Therefore, we describe some of the routinely used chemical processes to synthesize glycolide and lactide below.
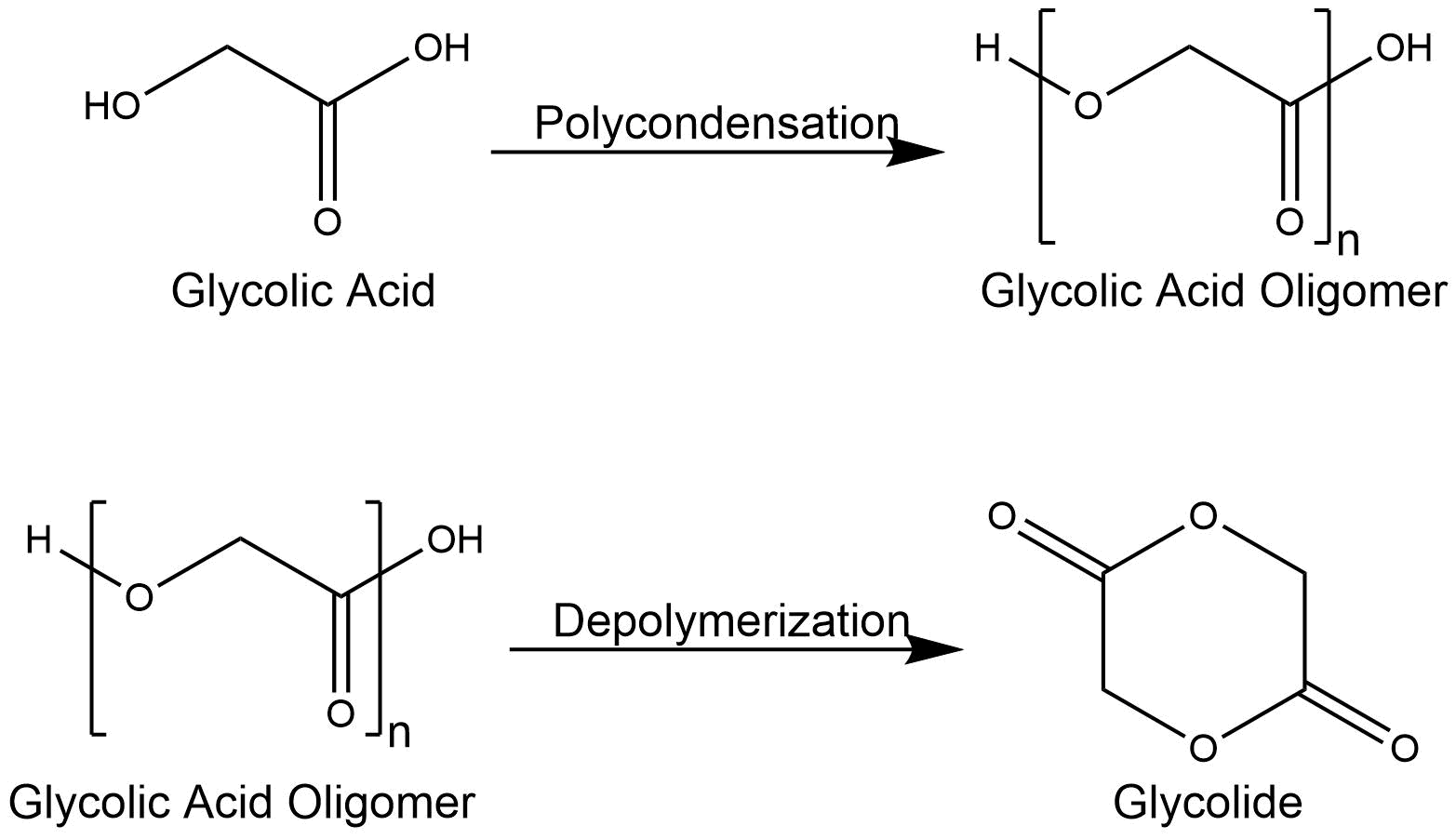
Glycolide
Glycolic acid is chemically synthesized, among other substrates, from formaldehyde, carbon monoxide and water. To dimerize the monomers to glycolide, glycolic acid is polymerized to glycolic acid oligomers via polycondensation. These oligomers possess a relatively small molecular weight of less than 20 kDa. They are subsequently depolymerized to glycolide dimers using tin catalysators, e.g. tin dichloride (SnCl2) or tin octanoate (Sn(Oct)2). [8]
Figure 2: Mechanism of glycolic acid dimerization
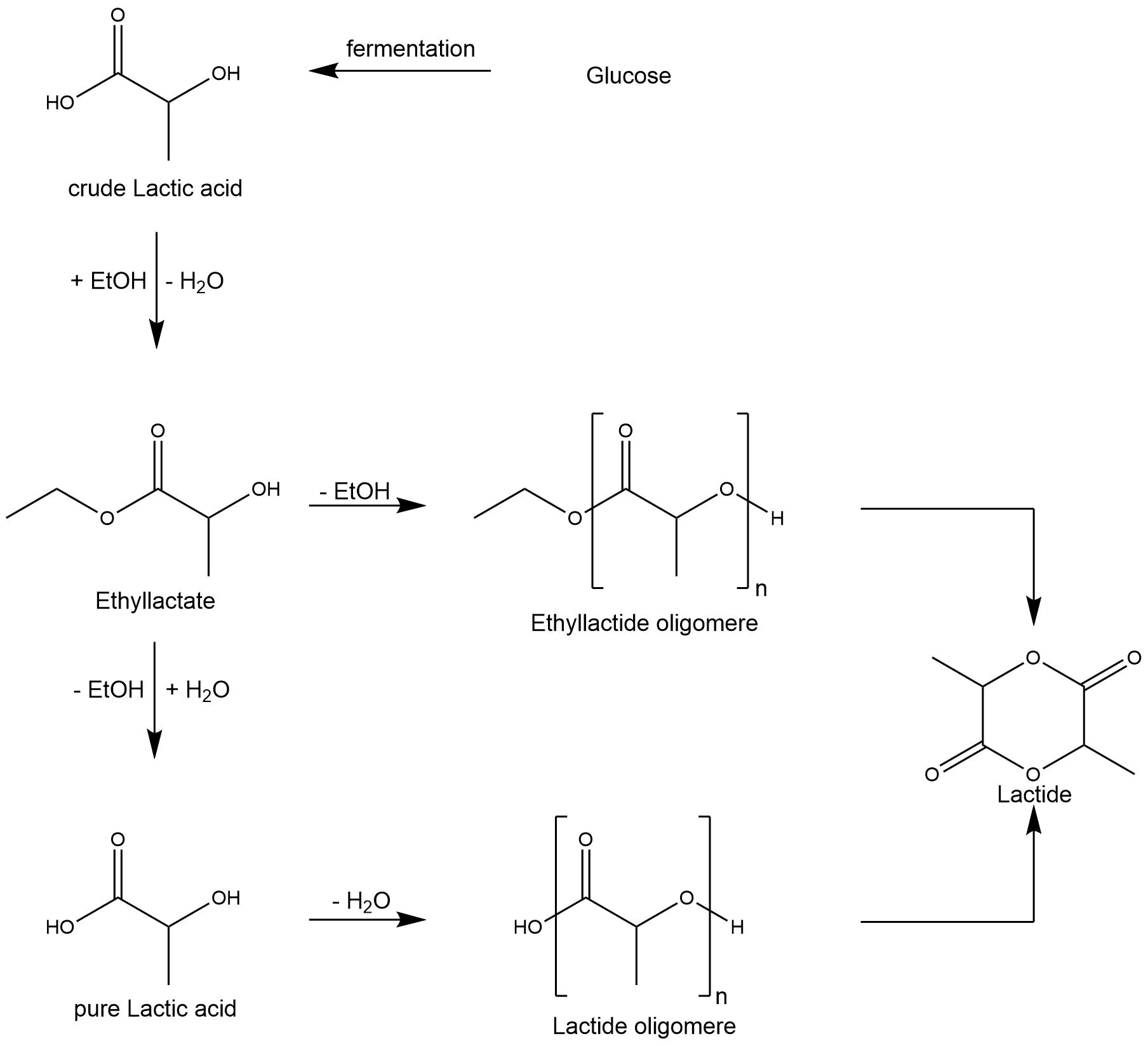
Lactide
Lactic acid is often produced by fermenting glucose, but its purity suffers from high levels of contaminating residual sugars or other organic acids. Since lactic acid is nonvolatile and easily oligomerizes at temperatures above 150 °C, distilling the fermentation broth in order to get pure lactic acid is not feasible. However, to circumvent the described impurities, impure lactic acid is esterified with alcohols to alkyl lactate, which can then be separated via distillation. The achieved alkyl lactate is then hydrolyzed to pure lactic acid. [9] Afterwards, the pure lactic acid is dimerized at high temperature and atmosphere pressure with help of a tin(II) oxide (SnO) catalyst to L-lactide following a similar depolymerization scheme as described in the glycolide section above. [9] This process is expensive due to high amounts of energy necessary in the distillation procedure and the large and complex distillation columns. However, Upare et al. investigated the direct conversion of alkyl lactate to lactide using different alkyl lactate esters and different catalysts. They state that their approach enables the production of lactide with better yields than the widely used approach described above. [9]
Figure 3: Mechanism of lactic acid dimerization.
References
- ↑ Dischert et al., United States Patent Application Publication, Jul.12,2012[1].
- ↑ Engineering Escherichia coli for glycolic acid production from D-xylose through the Dahms pathway and glyoxylate bypass. [2].
- ↑ Stryer, Lubert(2002): Biochemistry, 5th Edition, New York: W H Freeman. [3]
- ↑ Homepage Evry [4]
- ↑ Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation[5].
- ↑ Advances in metabolic engineering of yeast Saccharomyces cerevisiae for production of chemicals[6].
- ↑ CDAC Erbetta et al., Synthesis and Characterization of Poly(D,L-Lactide-co-Glycolide) Copolymer, Journal of Biomaterials and Nanobiotechnology, 2012, 3, 208-225. [7]
- ↑ Suzuki et al., Method for producing glycolide, US Patent: US8722908B2, 01/19/2010. [8]
- ↑ 9.0 9.1 9.2 Upare et al., Synthesis of Lactide from Alkyl Lactate via a Prepolymer Route., Ind. Eng. Chem. Res. 2012, 51, 4837−4842. [9]