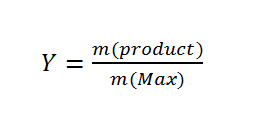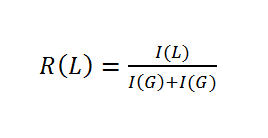Contents
PLGA
Abstract
There are two major methods for the synthesis of PLGA. The first method is the polycondensation reaction.[1] The second approach that we chose for our synthesis is the anionic ring opening polymerization (ROP). If the synthesis is performed using ROP we need a combination of an initiator and co-initiator to start a living polymerization. This has the advantage of producing higher molecular weights than the previous method, due to there is no release of water as a couple product. High molecular weights are important for our desired application. The downside of the ROP is that it can be killed with low amounts of impurities like water present. The reaction therefore has to be carried out under an inert gas atmosphere and impurities have to be removed before the reaction by applying a strong vacuum on the reaction vessel. This can be easily achieved by using a schlenk. The Polymer is later analyzed via gel permeation chromatography (GPC) and nuclear magnetic resonance (NMR) spectroscopy.[1]
The schlenk line
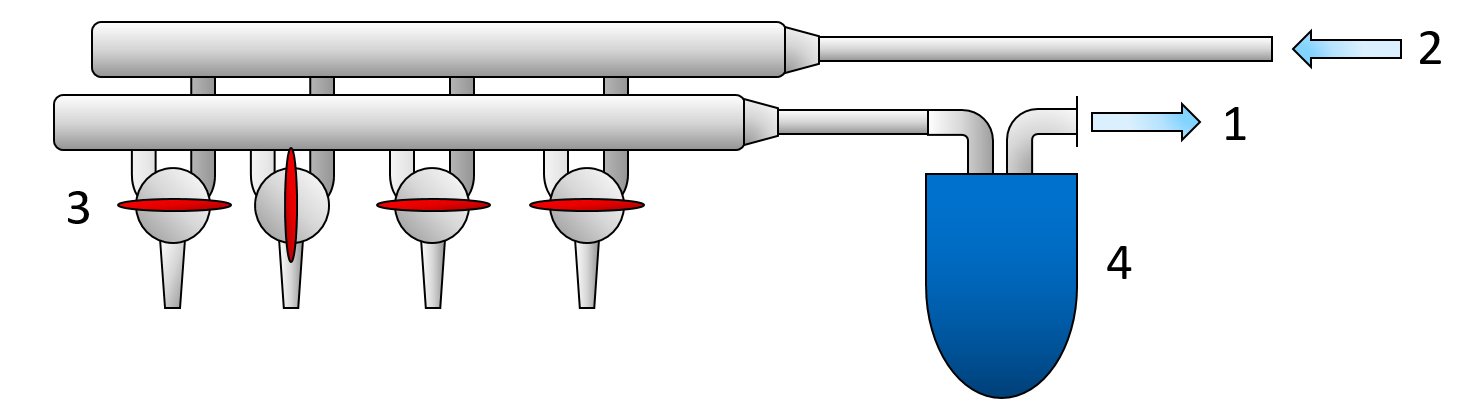
The schlenk line is a commonly used apparatus in chemical labs, that enables a quick change between pulling vacuum on the reaction vessel and applying an inert gas atmosphere. The change between inert gas and vacuum can occur in an instant by turning a special stopcock or a Teflon tap. The vacuum is applied using a vacuum pump. The pump has to be protected by an ice trap, that condenses any escaping moisture from the reaction vessel to prevent damage to the vacuum pump.
Figure 1: Illustration of a schlenk line. 1 connection to the vacuum pump. 2 connection to the inertgas source. 3 Stopcocks or a Teflon tap. 4 Ice trap
Synthesis of PLGA
Mechanism of the Anionic Ring Opening Polymerization
The anionic ring opening polymerization (ROP) is a polymerization mechanism where an initiator and a co-initiator are used to start formation of polymer chains. For our mechanism we are using stannous octate [Sn(Oct)2] as the initiator and octadecanole as co-initiator.
Figure 2 (left): Structure of stannous octoate
Figure 3 (right): Structure of octadecanol
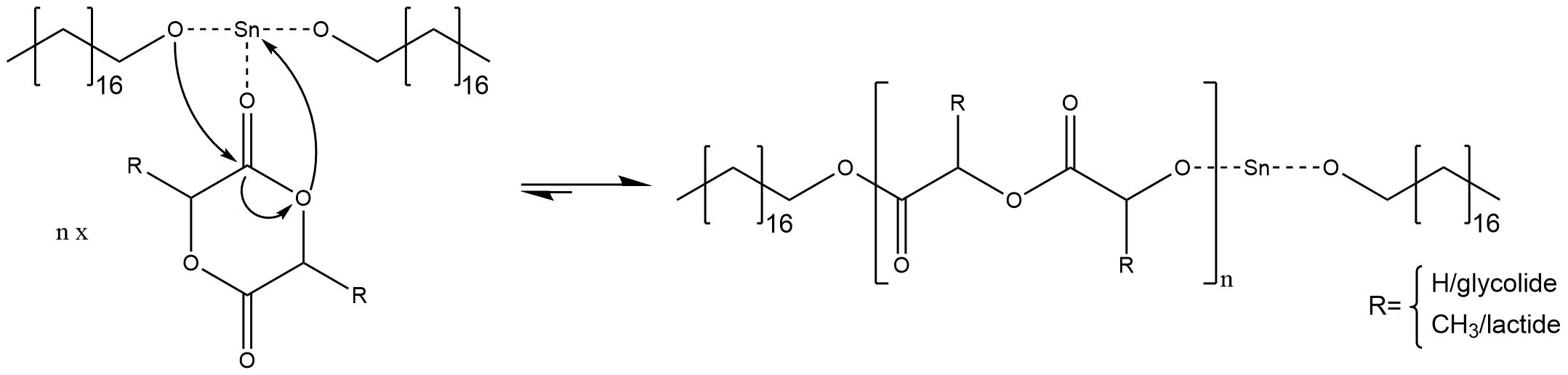
Befor the polymerisation reaction starts the initiator and co-initator react with eachother forming an alkoxide ion[2] [3] (figure 4)
The reaction starts after the initiator and co-initiator are added to the reaction vessel. First the metal ion of the initiator coordinates on the double bonded oxygen of the cyclic ester, which further increases the nucleophilic character of the carbon atom. Then the alcoholate ion of stannous octoate attacks the carbon atom forming a tetraehedral intermediat. The intermediate collapses instantly which opens up the cyclic ester and regenerates the tin alkoxide[2] [3] (figure 5).
Figure 5: Mechanism of the anionic ring opening polymerization.
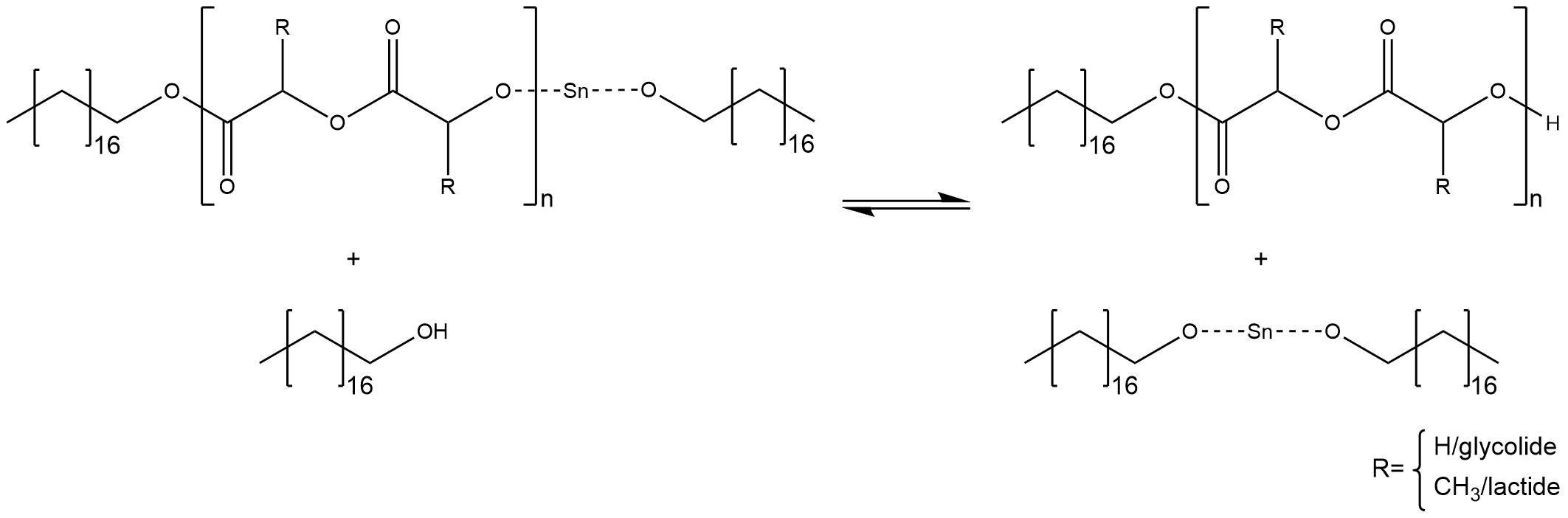
The repetition of this step leads to the growth of the polymer chains. Since there are no terminating steps involved, the chain growth has to be stopped manualy, by adding any alkohol e.g. methanole.
Figure 6: Termination reaction.
The anionic ring opening polymerization was achieved by melting the monomers. A strong vaccum was apllied to eliminate any remaining moisture in the reaction vessel, to prevent the deactivation of the initiator[2] [3] (Figure 6).
Controlling the molecular weight
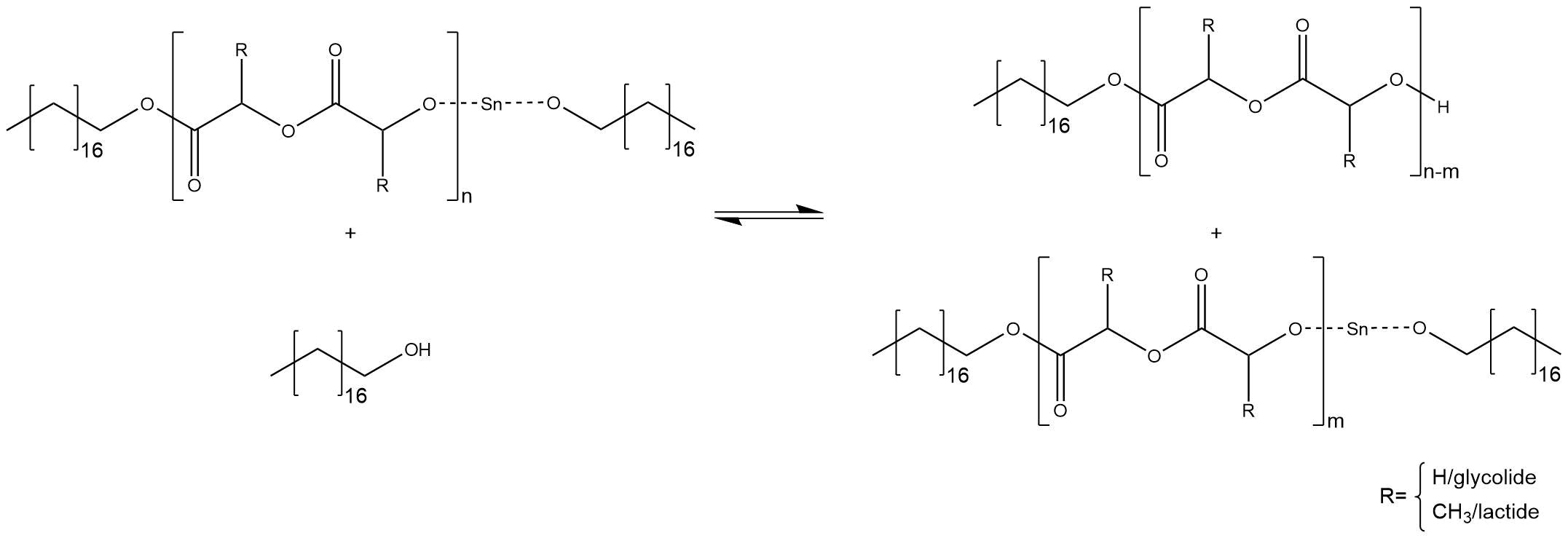
One activated co-initiator starts one growing chain. If the stoichiometric ratio between initiator and co-initiator is just right, there is no excess of the alcohol and all alcohol molecules are converted into the reactive stannous alkoxide. Statistically, every single chain grows with the same velocity until the monomers are completely converted into polymer. Thus, it is possible to calculate the amount of monomers inside the polymer chains, which should have nearly the same size. An increase of the ratio between initiator to monomer causes smaller chains and, in reverse, a decrease of the ratio causes bigger molecular weights. Therefore, it is important to stress that a predictable molecular weight needs a conversion of 100 %. If there is an excess of the co-initiator through using more co-initiator or less initiator, some free alcohol molecules can cause side reactions. The alcohol attacks the tin-alkoxide at the end of a chain and substitutes the proton of the hydroxyl group, regenerating the free active co-initiator, as shown in figure 4[2] [3].
Figure 7: Side reaction at the end of a chain, caused by free alcohol molecules.
The chain cannot grow further, the co-initiator can start a new chain. Another side reaction is a substitution inside a chain, which breaks down the chain into two smaller chains, as shown in figure 5[2] [3].
Figure 8: Side reaction inside a chain, caused by free alcohol molecules.
The chain, which bonds the tin alkoxide, can grow further. The main problem of these side reactions is the fact that chains with different sizes are synthesized. Therefore, the right ratio between initiator and co-initiator is essential for a predictable molecular weight.
Another important aspect of the synthesis is the fact that the glycolide monomers are more reactive than the lactide monomers. Glycolide has one methyl group less than lactide. Therefore it is easier to attack glycolide because the carbonyl carbon atom is less stericaly hindered. That can lead the glycolide to accumulate to blocks inside the polymer and the ratio of glycolide inside the polymer can be higher than the ratio of inserted monomers[1]. To prevent such blocks, it is necessary to have a homogeneous reaction medium with strong stirring and conversion rates on nearly 100%. If PLGA has glycolide blocks inside, it influences the properties. The solubility of PLGA is worse, the cristallinity is higher and the polymer degrades faster. Thus, it is important to guarantee a random distribution of monomers to prevent monomer blocks in the polymer chains.
Two set ups of PLGA were processed during our time in the laboratory through an anionic ring-opening polymerization. For that the monomers are put in a water free reaction vessel, melted and reacted with the initiators. The method is described in the method book.
PLGA (I) with a ratio lactide/glycolide of 75%/25%
and
PLGA (II) with a ratio lactide/glycolide of 67%/33%
Degradation of PLGA
Earlier it was mentioned, that PLGA is a biodegradable plastic. The reason why PLGA is biodegradable, whereas many other plastics are not, is the special structure. Plastics without any reactive areas or bonds on its polymer chains, like saturated hydrocarbon polymers or worse polyfluorinated polymers are not biodegradable. These cannot degrade, because the water or enzymes of microorganisms do not have the possibility to cleave any bonds inside. Polyesters like PLGA have a backbone with ester groups in regular intervals. Each one of these ester bonds can be cleaved by water or by enzymes.
To stay for PLGA, the degradation speed mainly depends on the ratio of monomers and the form of the polymer. Water has to diffuse inside the polymer to reach the reactive ester bonds. Therefore, a porous surface with gaps eases the diffusion of water, which causes a swelling of the polymer. After swelling the polymer starts to degrade into oligomers. The mechanism of the degradation is an ester hydrolysis. The ratio of monomers is the property largely influencing the degradation. Lactide has two more methyl groups than glycolide. This has two consequences. First, the methyl groups are non-polar. Thus, they shield the polymer from water and water needs more time to penetrate the polymer structure. Accordingly, it applies the more lactide there is inside the polymer, the longer the time of degradation. The second reason is, that the carbonylic carbon atom of lactide is near by the methyl group. Therefore, water has less space to attack and the possibility of hydrolysis is smaller than in glycolide. The oligomers further degrade into glycolic acid and lactic acid. Microorganisms can convert lactic acid and glycolic acid to CO2 and H2O. The form of the polymer influences the degradation through the size of its surface. If the polymer has a huge surface, there is more space for water to diffuse inside the polymer. However, if the density of the polymer is high, the surface of the polymer is smaller in relation to the mass of the polymer and it becomes more difficult for water to penetrate.[4]
Results and Discussion
GPC Results
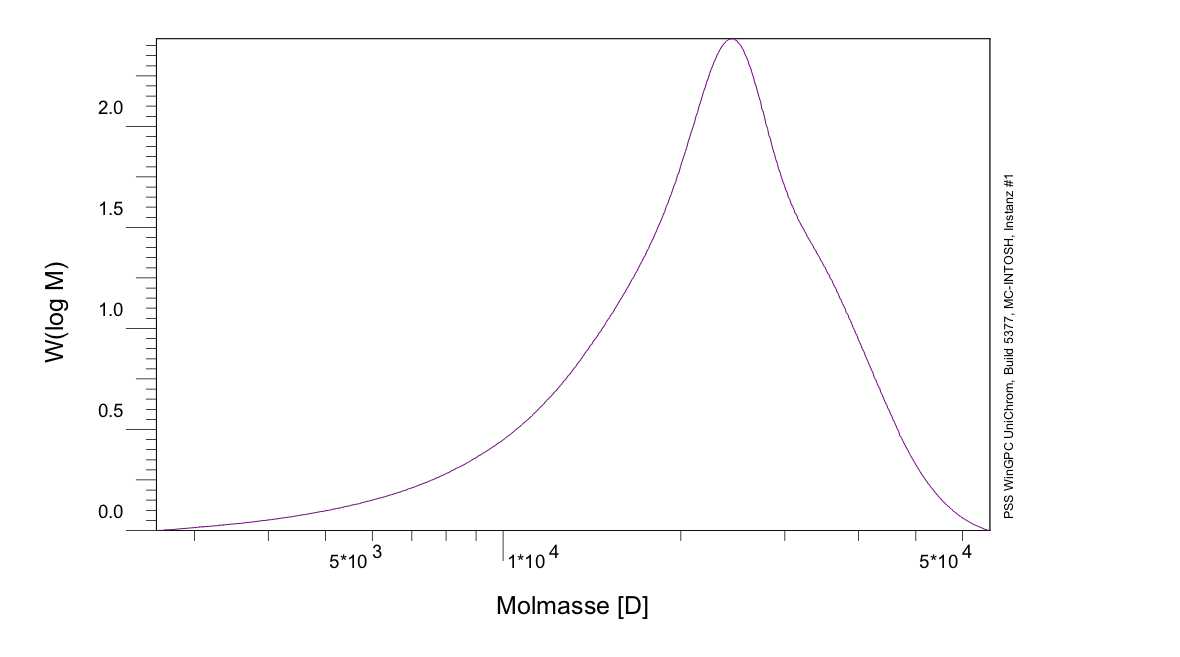
The expected molecular weight of PLGA (I) (ratio 75%/25%) was determined to be 361,669 g/mol, while the result of the GPC shows a molecular weight of 23,695 g/mol, as seen in figure 6, which means, that the chains are shorter than expected. Contrary to that the expected molecular weight of PLGA (II) (ratio 67%/33%) was determined to be 2.106*10^6 g/mol, while the GPC result shows a molecular weight of 167.9*10^6 g/mol, which means, that in this case the chains are longer than expected. During an informative dialog with Evonik, they gave us the hint, that the magnetic stirring mechanism, we were using, is not sufficient enough to stir the reaction mix at higher viscosity. This leads to a affectation of the growth of the polymer chains, while the reaction mix solidified. To avoid this effect, more sufficient stirring mechanism is necessary.
Figure 9: GPC of PLGA (I)
Furthermore they gave us the hint that the molecular weight, which was higher than expected, results on a deactivation of initiator molecules by water, leading the monomers to spread on less polymer chains, increasing their length. This can be avoided by working in an even more water-freed environment.
This improvements however are not possible to be implememted in our laboratory set up, because, we are able to work either with sufficient stirring devices or in a water free environment.
NMR-Results
After the polymer was purified and dried, the yield of the synthesis was determined.
with 1 \quad 2 Y 1 \qquad 2 yield
m 1 \qquad 2 mass
m(max)1 \qquad 2 maximal possible yield
The maximal possible yield is approximately equal to the total mass of monomers:
Table 1: Used amounts of monomers and yields of synthesis (total and relative).
| Polymer | m (lactide) [g] | m (glycolide) [g] | Total Mass [g] | Total Yield [g] | Relative Yield [%] |
|---|---|---|---|---|---|
| PLGA (I) | 10.810 | 2.901 | 13.7 | 2.703 | 19,72 |
| PLGA (II) | 13.13 | 5.26 | 18.43 | 3.306 | 17.94 |
For PLGA (I) we purified 100 % of our product and calculated the yield (shown in table 1). Since PLGA (II) was further used for nanosphere synthesis, total yield could not be determined.
As the yields in table 1 show, the relative yields are with 17.94% and 19.72% just about one fifth of the initial amount. This is caused by the increase of viscosity during synthesis, which makes magnetic stirring insufficient. Reactions were terminated when magnetic stirring stopped for reasons of reproducibility. To allow longer reaction times mechanical stirring would be necessary, which was unfortunately not available to us.
NMR-Spectroscopy:
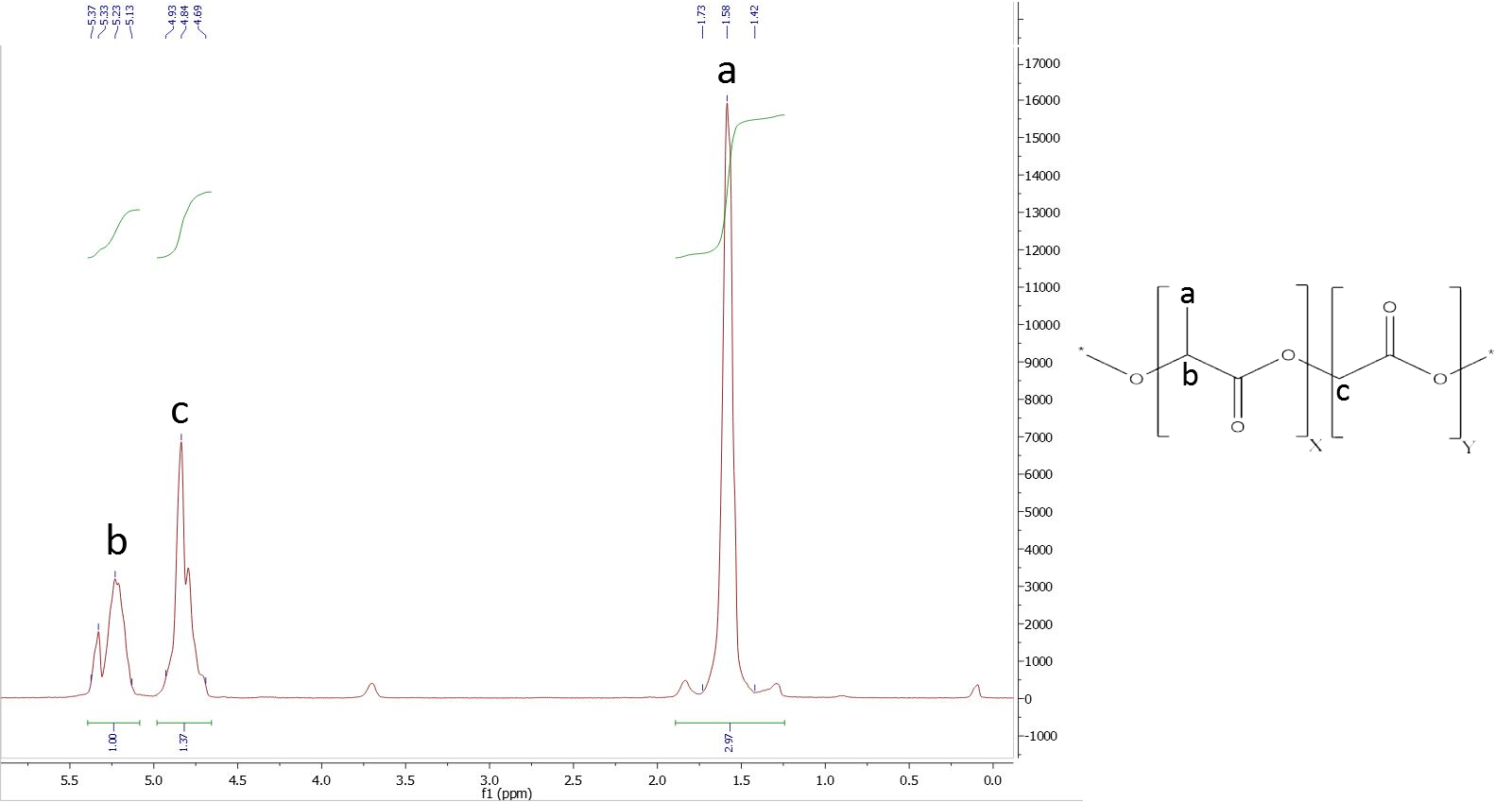
To analyze the ratios of our PLGA polymer, we used 1H NMR spectroscopy. We normalized the integral of the CH-group of lactide to 1.
To determine the composition, it is necessary to assign a peak to at least one specific proton group of each monomer. Figure 1 shows the structure of PLGA and the obtained NMR spectrum, which is a typical PLGA spectrum. The peaks used for calculation of monomer ratios belong to the methyl protons of lactide with a shift of δ=1.44-1.69 ppm, and the glycolide protons with a shift of δ=4.69-4.93 ppm (Figure 7).
Figure 10: NMR of PLGA (I)
Ratios of incorporated monomers were than computed with equation 2:
The NMR spectrum reveals that the ratio of incorporated monomers in the synthesized polymer does not correspond to initial monomer concentrations. . The final amount of glycolide in PLGA is higher, supporting the conclusion that glycolide is much more reactive than lactide.
Table 2: Mole fractions of inserted monomers compared to the mole fraction inside the produced polymer. Here it is visible, that the ratios of the start and the end of the reaction are different. Moreover, an increase of the initial glycolide amount of about 10 % results in an almost two-fold gain in the final ratio.
| Polymer | Inserted monomer ratio [L/G] in % | Monomer ratio after synthesis [L/G] in % |
|---|---|---|
| PLGA (I) | 75/25 | 59.13/40.87 |
| PLGA (II) | 66.6/33.3 | 38.76/61.24 |
The initial concentration of glycolide has significant impact on the ratio in the resulting polymer. If the amount is increased from 25 to 33%, the ratio of incorporated monomers (L/G) switches from about 60/40 to 40/60.
To predict the resulting relations, despite these differing reactivities, we tried to determine the rate constant for one monomer by using the obtained results. With these rate constants, a kinetic model was designed that is able to calculate ratios inside the polymer, depending on the relative amounts of monomers used for synthesis. Furthermore, this model allows the prediction of glycolide blocks. Glycolide blocks cause a deterioration of solubility, if the initial ratio of the synthesis is 1:1. For further information about our modeling approach see here.)
Outlook
PLGA and PLGC were produced using an insufficient magnetic stirring mechanism, which was not able to stir the reaction mixture at higher viscosity up to the end of the reaction. That led to a low conversion, which makes the results hardly reproducible. To avoid that, the reaction vessel needs to be designed in a way, which provides a continuous stirring all time throughout the reaction. The biggest difficulty lies in the achievement being able to have a mechanical stirring mechanism and pulling all humid atmospheric air out of the reaction vessel. Those conditions are not achievable with the laboratory equipment, which we can afford. With both conditions given, we would be able to get better reproducible results and more constant chain lengths. And with the polymers produced that way we would be also able to add additives and produce for example composite materials out of them.- ↑ 1.0 1.1 1.2 Cynthia D’Avila Carvalho Erbetta1, Ricardo José Alves2, Synthesis and Characterization of Poly(D,L-Lactide-co-Glycolide) Copolymer, Journal of Biomaterials and Nanobiotechnology,2003.
- ↑ 2.0 2.1 2.2 2.3 2.4 Yodthong Baimark and Robert Molloy, Synthesis and Characterization of Poly(L-lactide-co-ε-caprolactone) Copolymers:Effects of Stannous Octoate Initiator and Diethylene Glycol Coinitiator Concentrations, ScienceAsia, 2004 .
- ↑ 3.0 3.1 3.2 3.3 3.4 Adam Kowalski, Andrzej Duda, Mechanism of Cyclic Ester Polymerization Initiated with Tin(II) Octoate. 2. Macromolecules Fitted with Tin(II) Alkoxide Species Observed Directly in MALDI-TOF Spectra, Macromolecules, 2000 .
- ↑ Hirenkumar K. Makadia and Steven J. Siegel, Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier, Polymers 2011 .