|
|
| (25 intermediate revisions by 3 users not shown) |
| Line 190: |
Line 190: |
| | "> | | "> |
| | <div id="#Intro"> | | <div id="#Intro"> |
| − | <h3>Hardware</h3>
| |
| | <a class="anchorOffset" id="Intro"></a> | | <a class="anchorOffset" id="Intro"></a> |
| | <h4>Introduction</h4> | | <h4>Introduction</h4> |
| | | | |
| | | | |
| − | <p>To address the lack of accessibility to synthetic biology due to the high costs and the level of knowledge required among others, our team has designed Printeria, a fully-equipped bioengineering device capable of automate the process of printing genetic circuits in bacteria. | + | <p>To address the <b>lack of accessibility</b> to synthetic biology due to the high costs and the level of knowledge required among others, our team has designed <b>Printeria</b>, a fully-equipped bioengineering device capable of automate the process of printing genetic circuits in bacteria. |
| | </p> | | </p> |
| | | | |
| − | <p> To ensure a functional, economical and easy-to-use design, collaboration between the hardware and the wet lab teams has been necessary throughout the development process. After several drafts we have achieved a unique design that can be easily transported and replicated at a cost not exceeding 1.030,02 € (1.192,92 $).</p> | + | <p> To ensure a functional, economical and easy-to-use design, collaboration between the hardware and the wet lab teams has been necessary throughout the development process. After several drafts we have achieved a unique design that can be <b>easily transported</b> and <b>replicated</b> at a cost not exceeding 1.030,02 € (1.192,92 $).</p> |
| | | | |
| | <div style=" | | <div style=" |
| Line 212: |
Line 211: |
| | margin-bottom: 0.8em; | | margin-bottom: 0.8em; |
| | border-radius: 0.3em; | | border-radius: 0.3em; |
| − | width:350px | + | width:450px |
| | "> | | "> |
| | </a> | | </a> |
| Line 219: |
Line 218: |
| | width: 100%; | | width: 100%; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | padding-left: 16em; | + | padding-left: 18em; |
| − | "><h6 style="text-align: left">Printeria</h6> | + | "><h6 style="text-align: left">Image 1. Printeria</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| | | | |
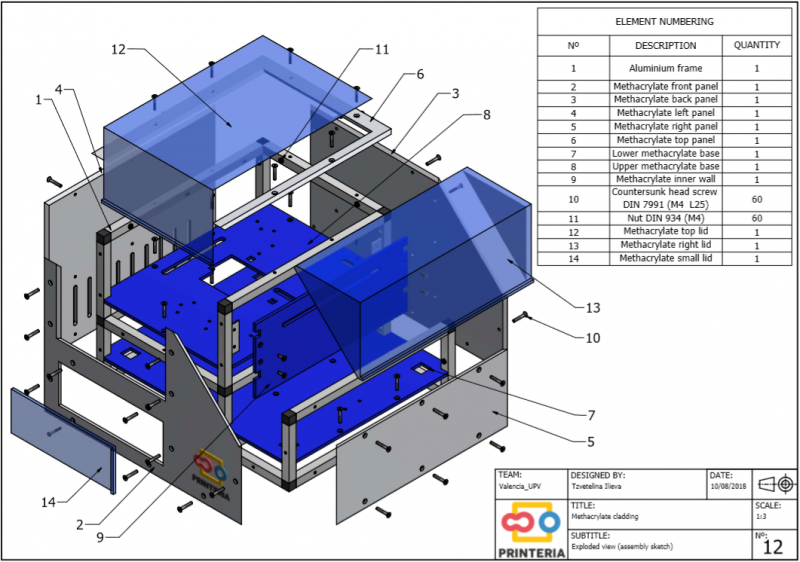
| − | <p> Printeria is divided into three main parts dependent on each other:</p> | + | <p> Printeria is divided into <b>three main parts</b> dependent on each other:</p> |
| | | | |
| | <div> | | <div> |
| Line 257: |
Line 256: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>Assembly Sketch</h6> | + | "><h6>Image 2. Assembly Sketch</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| | | | |
| − | <p> The software team has developed an intuitive <a href="https://2018.igem.org/Team:Valencia_UPV/Software" target="_blank">software</a> that controls the operation of Printeria and allows the user to easily design the genetic circuit that is going to be printed in the machine.</p> | + | <p> The software team has developed an intuitive <a href="https://2018.igem.org/Team:Valencia_UPV/Software" target="_blank">software</a> that controls the operation of Printeria and allows the user to easily design the genetic circuit that is going to be printed.</p> |
| | | | |
| | <ul class="accordion accordion-1" style=""> | | <ul class="accordion accordion-1" style=""> |
| Line 339: |
Line 338: |
| | <h5 style="padding-left: 1em;">Frame</h5> | | <h5 style="padding-left: 1em;">Frame</h5> |
| | <div style="padding-left: 1em;"> | | <div style="padding-left: 1em;"> |
| − | <p>First of all, it is necessary to design a structure that confines all the elements necessary for the operation of the device inside it and also allows the creation of a sterile atmosphere to carry out the biological reactions.</p> | + | <p>First of all, it is necessary to design a structure that confines all the elements necessary for the operation of the device inside it and also allows the creation of a <b>sterile atmosphere</b> to carry out the biological reactions.</p> |
| | | | |
| − | <p> In order to facilitate its transport, aluminum has been chosen for the frame, which in addition to its low weight, has other great qualities such as great resistance to corrosion and low cost.</p> | + | <p> In order to facilitate its transport, <b>aluminum</b> has been chosen for the frame, which in addition to its low weight, has other great qualities such as great resistance to corrosion and <b>low cost</b>.</p> |
| | | | |
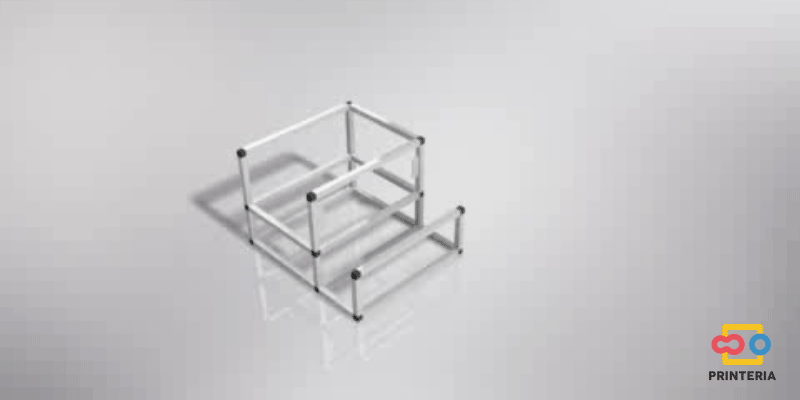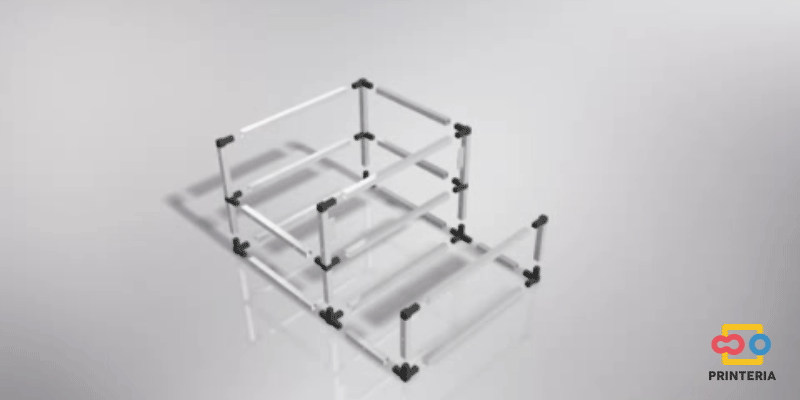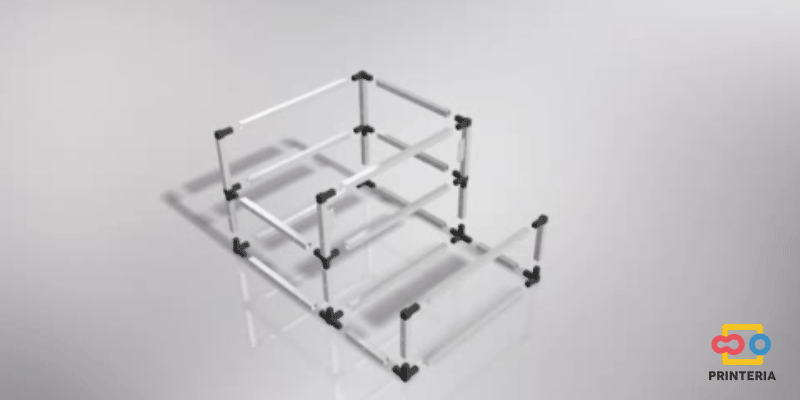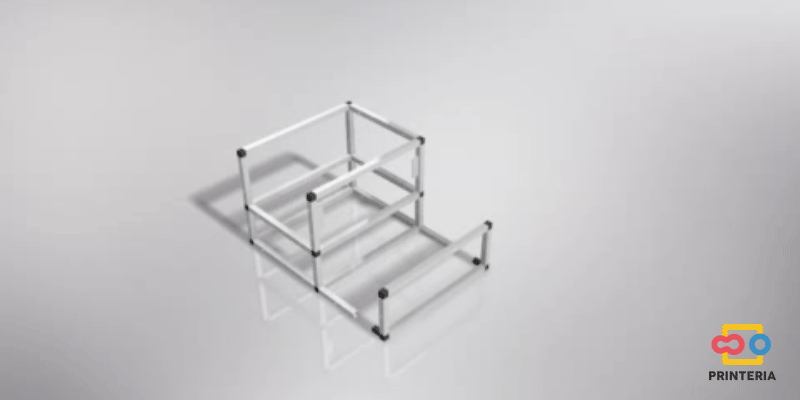
| − | <p> It has been used 15x15x1mm square tube profiles joined by plastic corner connectors obtaining as a result a 415x360x280mm chassis. Thanks to the generated blueprints we have been able to contact a company specializing in cutting and handling aluminum that has provided us with all the parts as we required. As it was not possible for us to find plastic connectors that fit our needs, we have decided to design these parts ourselves and then print them on a 3D printer. </p> | + | <p> It has been used 15x15x1mm square tube profiles joined by plastic corner connectors obtaining as a result a 415x360x280mm chassis. Thanks to the generated <b>blueprints</b> we have been able to contact a company specializing in cutting and handling aluminum that has provided us with all the parts as we required. As it was not possible for us to find plastic connectors that fit our needs, we have decided to design these parts ourselves and then print them on a 3D printer. </p> |
| | | | |
| | <div style=" | | <div style=" |
| Line 363: |
Line 362: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6 style="text-align: right;">Aluminium frame</h6> | + | "><h6 style="text-align: right;">Image 3. Aluminium frame</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 371: |
Line 370: |
| | | | |
| | <div style="padding-left: 1em;"> | | <div style="padding-left: 1em;"> |
| − | <p> To complete the structure, it is necessary to create walls that are attached to this frame and contribute to its stability. We have opted for the use of methacrylate due to its attractive appearance, durability and scratch resistance. </p> | + | <p> To complete the structure, it is necessary to create walls that are attached to this frame and contribute to its stability. We have opted for the use of <b>methacrylate</b> due to its attractive appearance, durability and scratch resistance. </p> |
| | | | |
| − | <p> During the process of designing the device several sketches were made and after consulting potential users of the machine it was concluded that the front lid of the device should be as large as possible to facilitate the feeding of consumables. In addition, it has been decided that this lid should be transparent in order to allow the user to observe the reaction if desired, thus being able to detect possible faults at a glance. </p> | + | <p> During the process of designing the device several sketches were made and after consulting potential users of the machine it was concluded that the <b>front lid</b> of the device should be as large as possible to facilitate the feeding of consumables. In addition, it has been decided that this lid should be <b>transparent</b> in order to allow the user to observe the reaction if desired, thus being able to detect possible failures at a glance. </p> |
| | | | |
| − | <p> On the other hand, the right lid that covers the output system is transparent to show what is inside but it should be completely opaque to prevent the entry of light and thus obtain better measurements of OD and Fluorescence.</p> | + | <p> On the other hand, the right lid that covers the output system is transparent to show what is inside but it should be <b>completely</b> opaque to prevent the entry of light and thus obtain better measurements of OD and Fluorescence.</p> |
| | | | |
| | <p> As well as the aluminum profiles, the blueprints created were used for ordering the custom-made parts to a company.</p> | | <p> As well as the aluminum profiles, the blueprints created were used for ordering the custom-made parts to a company.</p> |
| Line 396: |
Line 395: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6 style="text-align: right">Methacrylate cladding</h6> | + | "><h6 style="text-align: right">Image 4. Methacrylate cladding</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 402: |
Line 401: |
| | <p> Finally, 4mm diameter countersunk head screws DIN 7991 (M4 L25) and nuts DIN 934 (M4), both made of galvanized steel, were used for joining the methacrylate plates to the frame. </p> | | <p> Finally, 4mm diameter countersunk head screws DIN 7991 (M4 L25) and nuts DIN 934 (M4), both made of galvanized steel, were used for joining the methacrylate plates to the frame. </p> |
| | | | |
| − | <p> Thanks to the use of plastic connectors and bolted joints, the entire structure can be disassembled and reassembled as often as required, making it much easier to transport and store. </p> | + | <p> Thanks to the use of plastic connectors and bolted joints, the entire structure can be <b>disassembled and reassembled</b> as often as required, making it much easier to transport and store. </p> |
| | | | |
| | <div style=" | | <div style=" |
| Line 422: |
Line 421: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>Parts of the structure before assembling</h6> | + | "><h6>Image 5. Parts of the structure before assembling</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 441: |
Line 440: |
| | <div style=" | | <div style=" |
| | width: 100%; | | width: 100%; |
| − | padding-left: 19em; | + | padding-left: 17em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>Assembly sketch of the methacrylate cladding</h6> | + | "><h6>Image 6. Assembly sketch of the methacrylate cladding</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 452: |
Line 451: |
| | | | |
| | <div style="padding-left: 1em;"> | | <div style="padding-left: 1em;"> |
| − | <p> Given the great importance of keeping the reaction zone perfectly horizontal, it is necessary to use levelling feet. In addition, for checking the horizontal position, a bull’s eye level has been installed next to the reaction zone. </p> | + | <p> Given the great importance of keeping the reaction zone <b>perfectly horizontal</b>, it is necessary to use levelling feet. In addition, for checking the horizontal position, a bull’s eye level has been installed next to the reaction zone. </p> |
| | | | |
| | <div style=" | | <div style=" |
| Line 470: |
Line 469: |
| | <div style=" | | <div style=" |
| | width: 100%; | | width: 100%; |
| − | padding-left: 13em; | + | padding-left: 10em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6 style="text-align: left;">Leveling system</h6> | + | "><h6 style="text-align: left;">Image 7. Leveling system</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 593: |
Line 592: |
| | </div> | | </div> |
| | | | |
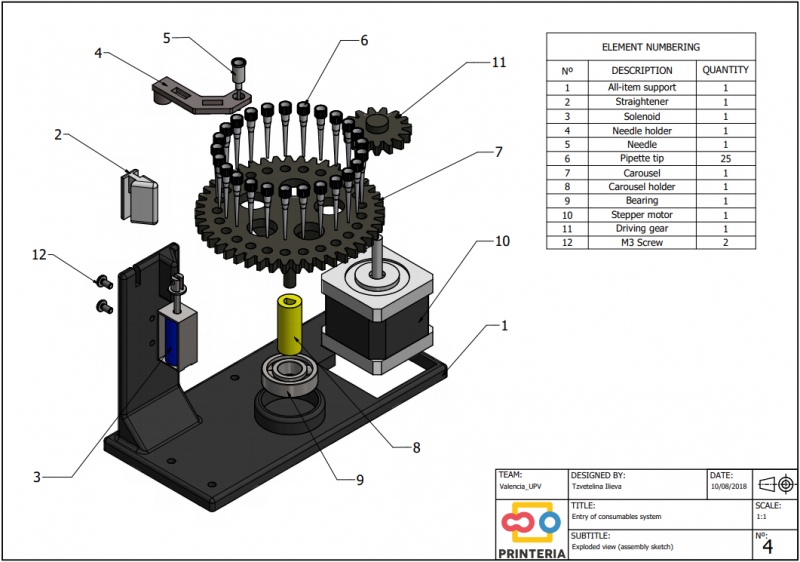
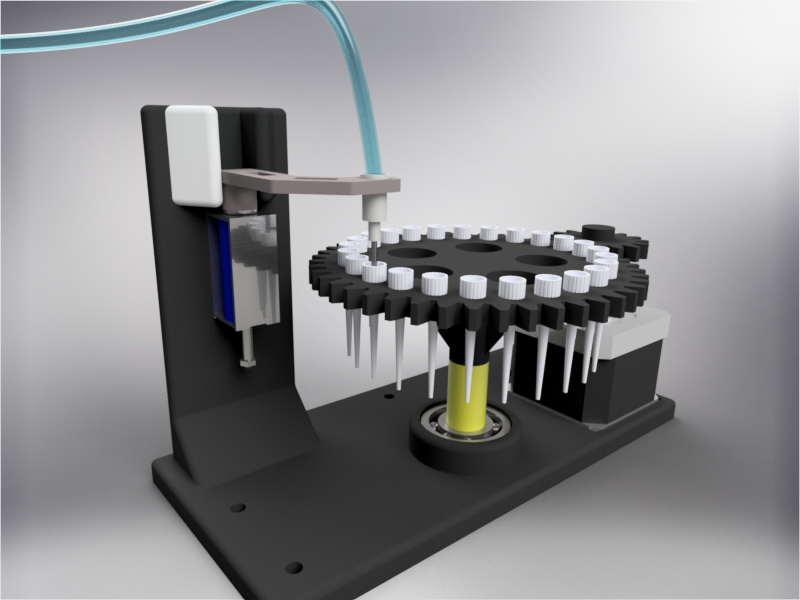
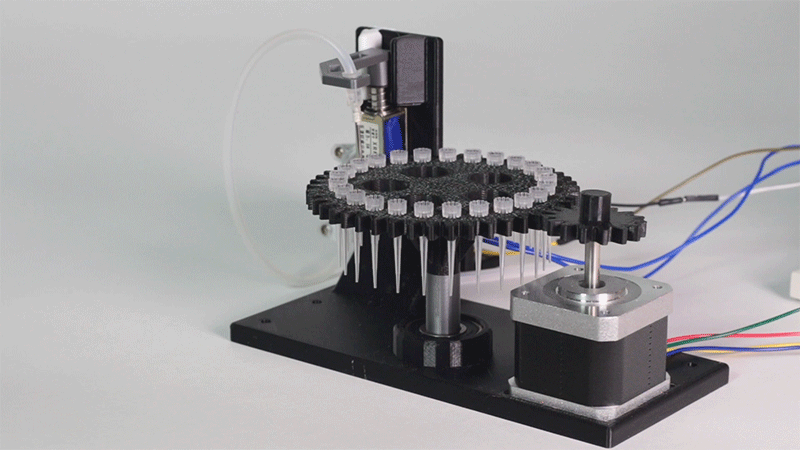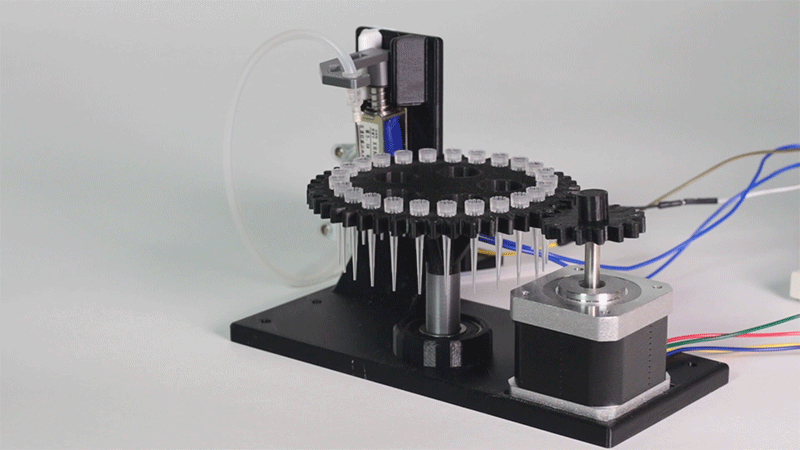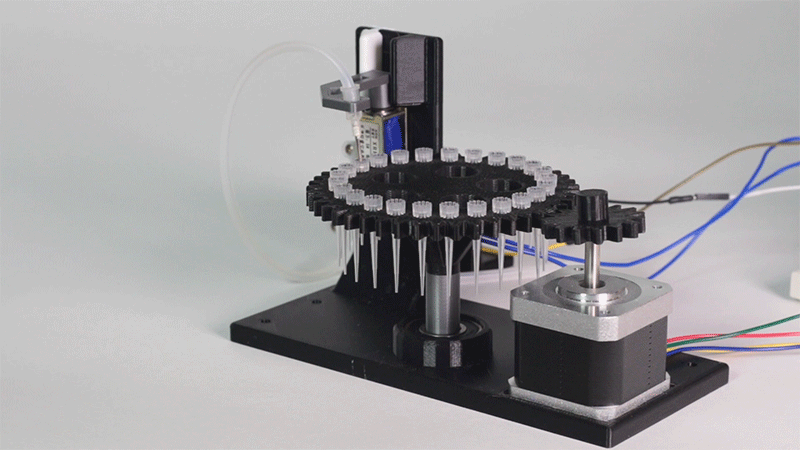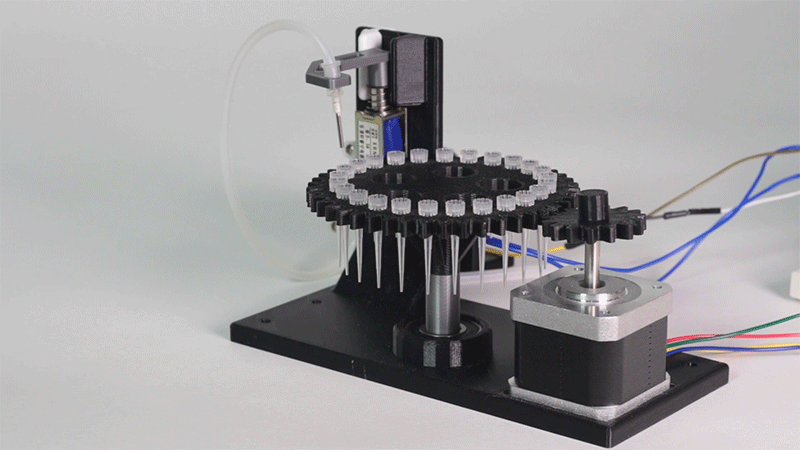
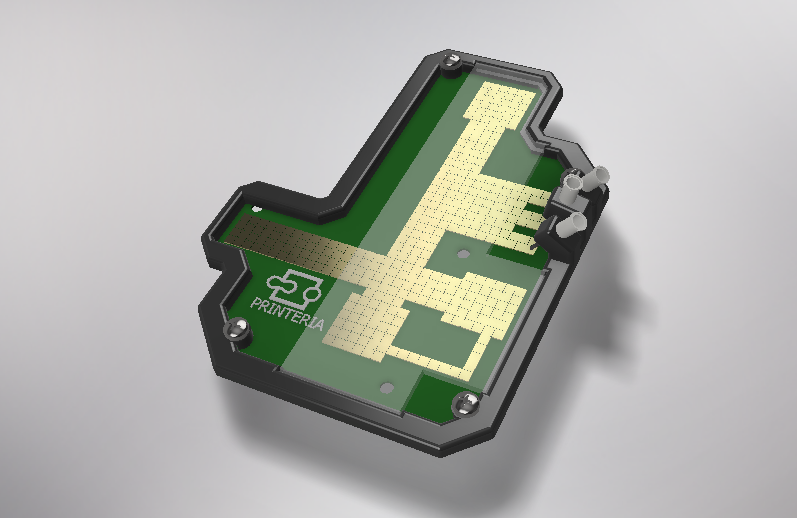
| − | <p>In order to carry out the reactions it is necessary to introduce the consumables into the PCB. Therefore, a carousel-based system has been designed in which pipette tips with the parts necessary for the reaction are placed. The user should place these pipette tips before the experiments in the positions indicated. </p> | + | <p>In order to carry out the reactions it is necessary to introduce the <b>consumables</b> into the PCB. Therefore, a <b>carousel-based system</b> has been designed in which pipette tips with the parts necessary for the reaction are placed. The user should place these pipette tips before the experiments on the positions indicated. </p> |
| | | | |
| | <div style=" | | <div style=" |
| Line 612: |
Line 611: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>Foto de todo el sistema de entrada</h6> | + | "><h6>Image 8. Entry system assembly sketch</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| | | | |
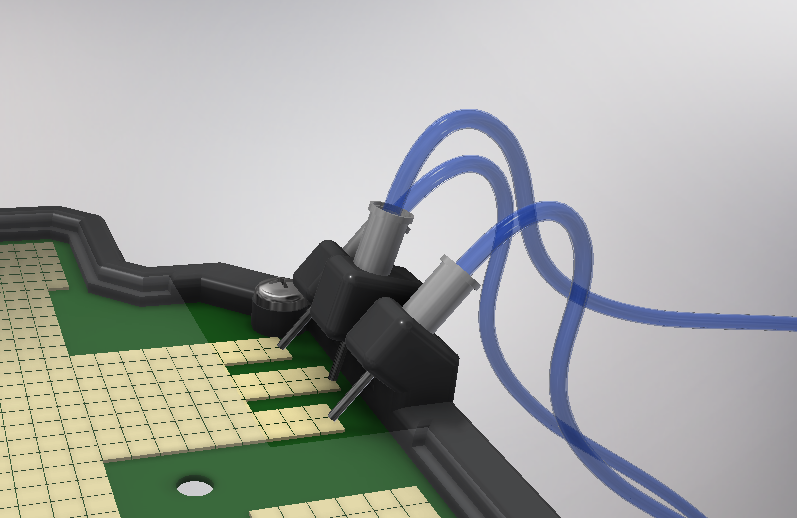
| − | <p> Once the tips have been inserted and the machine is ready to operate, the carousel is rotated with the help of a stepper motor until each of the tips is placed in the blowing position. In this position a needle connected to a peristaltic pump lowers vertically and injects air, thus producing the fall of the genetic material on the PCB. </p> | + | <p> Once the tips have been inserted and the machine is ready to operate, the carousel is rotated with the help of a stepper motor until each of the tips is placed in the <b>blowing position</b>. In this position a needle connected to a peristaltic pump lowers vertically and injects air, thus producing the fall of the genetic material on the PCB. </p> |
| | | | |
| − | <p> The vertically moving needle is made possible by the use of a solenoid, a device that converts electrical energy into mechanical energy which, in turns, raises or lowers the needle. Once the needle is in its low position and inserted into the pipette tip, the pump is activated and air is injected. </p> | + | <p> The vertically moving needle was made possible thanks to the use of a <b>solenoid</b>, a device that converts electrical energy into mechanical energy which, in turns, raises or lowers the needle. Once the needle is on its low position and inserted into the pipette tip, the pump is activated and air is injected. </p> |
| | | | |
| | <div> | | <div> |
| Line 650: |
Line 649: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>Needle in its high and low position</h6> | + | "><h6>Image 9. Needle on its high and low position</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| | </div> | | </div> |
| | | | |
| − | <p> All the necessary parts have been designed using a 3D modeling software and then printed on our 3D printer, which means that we have been able to implement this system at a very low cost. </p> | + | <p> All the necessary parts have been designed using a 3D modeling software and then printed on our <b>3D printer</b>, which means that we have been able to implement this system at a very <b>low cost</b>. </p> |
| | | | |
| | <div style=" | | <div style=" |
| Line 673: |
Line 672: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>Proof of concept of the input system</h6> | + | "><h6>Image 10. Proof of concept of the entry system</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 777: |
Line 776: |
| | <div style="padding-left: 1em;"> | | <div style="padding-left: 1em;"> |
| | | | |
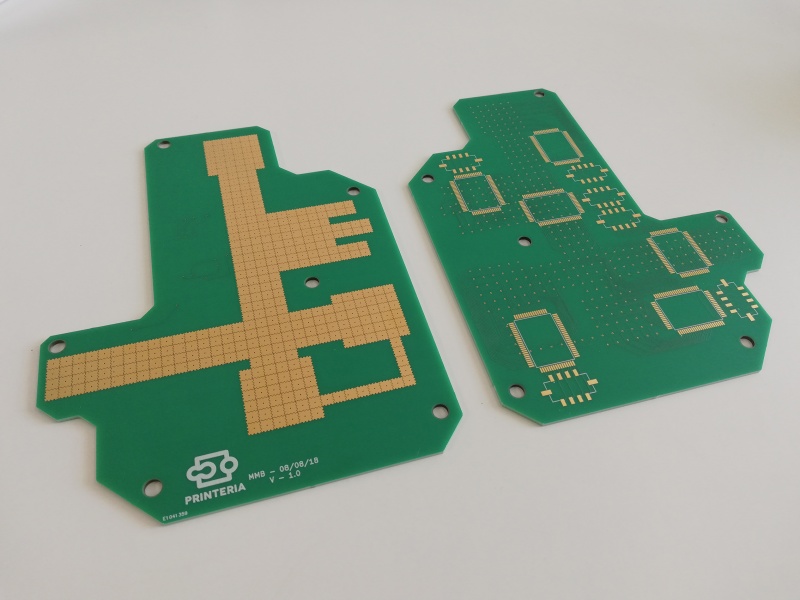
| − | <p>For the main part of the device we needed to be able to reliably and precisely control the movement of liquids involved in the reaction. This ensures that the components mix was intended and then follow every procedure accurately. For this we have decided to use a technology called digital microfluidics, which is based on applying electromagnetic fields to a special surface so that it changes its properties. More precisely we can change the hydrophobicity of the surface as to attract droplets and repel them, ensuring in this way that they follow an established route.</p> | + | <p>For the main part of the device we needed to be able to reliably and precisely <b>control the movement of the liquids</b> involved in the reaction. This ensures that the components mix was intended and then follow every procedure accurately. For this we have decided to use a technology called <b>digital microfluidics (DMF)</b>, which is based on applying electromagnetic fields to a special surface so that it changes its properties. More precisely we can <b>change</b> the <b>hydrophobicity</b> of the surface as to attract droplets and repel them, ensuring in this way that they follow an established route.</p> |
| − | <p>This enables us to mix the different components, split the mixture into separate droplets to control the volume and allows multiple reactions to take place in very little space thanks to this flexible technology.</p> | + | <p>This enables us to <b>mix</b> the different components, <b>split</b> the mixture into separate droplets to control the volume and allows multiple reactions to take place in very little space thanks to this flexible technology.</p> |
| | | | |
| | | | |
| Line 798: |
Line 797: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>DMF technology</h6> | + | "><h6>Image 11. DMF technology</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 805: |
Line 804: |
| | <div style="padding-left: 1em;"> | | <div style="padding-left: 1em;"> |
| | <a class="anchorOffset" id="finaldesign"></a> | | <a class="anchorOffset" id="finaldesign"></a> |
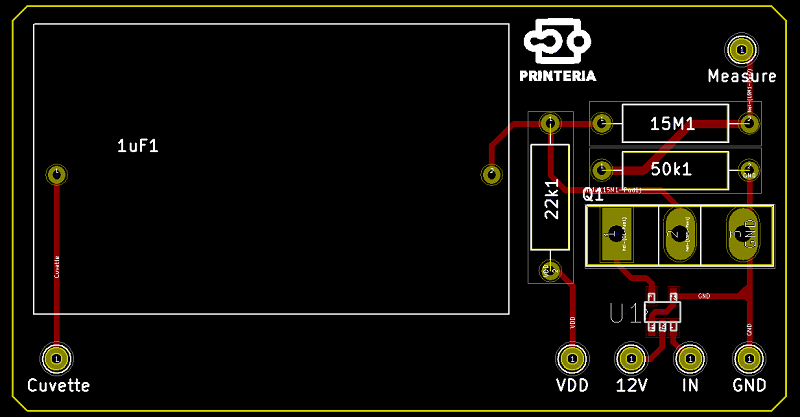
| − | <p>To take advantage of this we have designed the PCB under the surface with small pads on which we can apply a high voltage to cause this effect on the coating of our surface.</p> | + | <p>To take advantage of this we have designed the <b>PCB</b> under the surface with small pads on which we can apply a high voltage to cause this effect on the coating of our surface.</p> |
| | <div style=" | | <div style=" |
| | padding-bottom: 1em; | | padding-bottom: 1em; |
| Line 822: |
Line 821: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>PCB designed</h6> | + | "><h6>Image 12. PCB designed</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 828: |
Line 827: |
| | | | |
| | <div style="padding-left: 1em;"> | | <div style="padding-left: 1em;"> |
| − | <p>This surface must be very hydrophobic, therefore it is necessary to coat the PCB with a polytetrafluoroethylene (PTFE) foil (12 microns thickness) and then apply a thin layer of a very hydrophobic gel called FluoroPel. In order to keep the Teflon foil well glued to the surface of the PCB, a custom-made piece has been designed and printed. The foil will be glued to this piece and then joined to the PCB by means of screws.</p> | + | <p>This surface must be very hydrophobic, therefore it is necessary to coat the PCB with a polytetrafluoroethylene <b>(PTFE)</b> foil (12 microns thickness) and then apply a thin layer of a very hydrophobic gel called <b>FluoroPel</b>. In order to keep the Teflon foil well glued to the surface of the PCB, a custom-made piece has been designed and printed. The foil will be glued to this piece and then joined to the PCB by means of screws.</p> |
| | | | |
| | <div style=" | | <div style=" |
| Line 846: |
Line 845: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>PCB designed and its holder</h6> | + | "><h6>Image 13. PCB designed and its holder</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 852: |
Line 851: |
| | | | |
| | <div style="padding-left: 1em;"> | | <div style="padding-left: 1em;"> |
| − | <p>To be able to control so many pads we are using a HV507, a special driver chip which is a high voltage serial to parallel converter. This means that it takes data one bit at a time and then it displays them all at the same time at a high voltage. This chip can handle up to 64 outputs, meaning that we can connect 64 pads to it. So we only need a total of 6 chips for our entire surface.</p> | + | <p>To be able to control so many pads we are using a HV507, a special <b>driver chip</b> which is a high voltage serial to parallel converter. This means that it takes data one bit at a time and then it displays them all at the same time at a high voltage. This chip can handle up to 64 outputs, meaning that we can connect 64 pads to it. So we only need a total of <b>6 chips</b> for our entire surface.</p> |
| | </div> | | </div> |
| | <div style=" | | <div style=" |
| Line 870: |
Line 869: |
| | <div style=" | | <div style=" |
| | width: 100%; | | width: 100%; |
| − | padding-left: 10em; | + | padding-left: 7em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6 style="text-align: left ">Driver chips on the PCB</h6> | + | "><h6 style="text-align: left ">Image 14. Driver chips on the PCB</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 893: |
Line 892: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>Proof of concept of moving droplets</h6> | + | "><h6>Image 15. Proof of concept of moving droplets</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 899: |
Line 898: |
| | | | |
| | <div style="padding-left: 1em;"> | | <div style="padding-left: 1em;"> |
| − | <p>To get the droplets out of the reaction surface we have designed a holder for a syringe that has a tube with a pump connected to the experiment zone and the output recipient. For extracting the droplet we move it to the syringe tip on the pcb surface and then activate the pump. This absorbs the droplet, makes it pass between 2 metal plates that electroporate the droplet, and drops it on the output tube. This part of the device is also used for dispensing later the 3 ml of LB medium onto the tube. In addition, it is also used for getting the cleaning droplets out of the experimentation zone, while cleaning the tube itself.</p> | + | <p>To get the droplets out of the reaction surface we have designed a holder for a syringe that has a tube with a pump connected to the experiment zone and the output recipient. For <b>extracting the droplet</b> we move it to the syringe tip on the PCB surface and then activate the <b>pump</b>. This absorbs the droplet, makes it pass between 2 metal plates that <b>electroporate</b> it, and drops it on the output tube. This part of the device is also used for dispensing later the 3 ml of LB medium onto the tube. In addition, it is also used for getting the <b>cleaning droplets</b> out of the experimentation zone, while cleaning the tube itself.</p> |
| | | | |
| | <div style=" | | <div style=" |
| Line 916: |
Line 915: |
| | <div style=" | | <div style=" |
| | width: 100%; | | width: 100%; |
| − | padding-left: 11em; | + | padding-left: 8em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6 style="text-align: left">PCB output</h6> | + | "><h6 style="text-align: left">Image 16. PCB output</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 929: |
Line 928: |
| | <div style="padding-left: 1em;"> | | <div style="padding-left: 1em;"> |
| | | | |
| − | <p>For the Golden Gate assembly reaction we needed to make the droplets with the DNA and the enzymes go through a thermocycling process, which means to change the temperature in cycles. </p> | + | <p>For the Golden Gate assembly reaction we needed to make the droplets with the DNA and the enzymes go through a <b>thermocycling process</b>, which means to change the temperature in cycles. </p> |
| | | | |
| | <div class="divTabla"> | | <div class="divTabla"> |
| Line 949: |
Line 948: |
| | <td class="tdHec">3 min</td> | | <td class="tdHec">3 min</td> |
| | <td class="tdHec">37 ºC</td> | | <td class="tdHec">37 ºC</td> |
| − | <td rowspan="2" class="tdHec">2</td> | + | <td rowspan="2" class="tdHec">25</td> |
| | </tr> | | </tr> |
| | <tr> | | <tr> |
| Line 964: |
Line 963: |
| | </div> | | </div> |
| | | | |
| − | <h6> Thermocycling process </h6> | + | <h6>Table 1. Thermocycling process </h6> |
| | | | |
| − | <p>To take advantage of the digital microfluidics, instead of changing the temperature of a fixed place, we have decided to have the droplet alternating between two places that are at the correct temperature. This means that the temperature of the droplet changes very quickly. And it also means that we only have to stabilize two discrete temperatures instead of changing them constantly, which translates into greater temperature accuracy.</p> | + | <p>To take advantage of the digital microfluidics, instead of changing the temperature of a <b>fixed place</b>, we have decided to have the droplet <b>alternating between two places</b> that are at the correct temperature. This means that the temperature of the droplet changes very quickly. And it also means that we only have to stabilize two discrete temperatures instead of changing them constantly, which translates into <b>greater temperature accuracy</b>.</p> |
| − |
| + | |
| − | <div style="
| + | |
| − | padding-bottom: 1em;
| + | |
| − | text-align: right;
| + | |
| − | ">
| + | |
| − | <a href="https://static.igem.org/mediawiki/2018/thumb/3/3f/T--Valencia_UPV--PCB6UPV2018.jpeg/609px-T--Valencia_UPV--PCB6UPV2018.jpeg.png"
| + | |
| − | data-lightbox="true">
| + | |
| − | <img src="https://static.igem.org/mediawiki/2018/thumb/3/3f/T--Valencia_UPV--PCB6UPV2018.jpeg/609px-T--Valencia_UPV--PCB6UPV2018.jpeg.png" alt="" style="
| + | |
| − | margin-top: 0.5em;
| + | |
| − | margin-bottom: 0.8em;
| + | |
| − | border-radius: 0.3em;
| + | |
| − | ">
| + | |
| − | </a>
| + | |
| − | <div style="
| + | |
| − | width: 100%;
| + | |
| − | padding-left: 19em;
| + | |
| − | padding-right: 1em;
| + | |
| − | "><h6>Hot and cold zones on the PCB</h6>
| + | |
| − | </div>
| + | |
| − | </div>
| + | |
| | | | |
| − | <div style="
| + | <div style=" |
| | padding-bottom: 1em; | | padding-bottom: 1em; |
| − | text-align: right; | + | text-align: center; |
| | "> | | "> |
| − | <a href="https://via.placeholder.com/800x400" | + | <a href="https://static.igem.org/mediawiki/2018/e/ef/T--Valencia_UPV--PCBTERMOUPV2018.jpeg" |
| − | data-lightbox="true">
| + | data-lightbox="true"> |
| − | <img src="https://via.placeholder.com/800x400" alt="" style=" | + | <img src="https://static.igem.org/mediawiki/2018/e/ef/T--Valencia_UPV--PCBTERMOUPV2018.jpeg" alt="" style=" |
| | margin-top: 0.5em; | | margin-top: 0.5em; |
| | margin-bottom: 0.8em; | | margin-bottom: 0.8em; |
| | border-radius: 0.3em; | | border-radius: 0.3em; |
| | "> | | "> |
| − | </a>
| + | </a> |
| − | <div style=" | + | <div> |
| − | width: 100%;
| + | <div style=" |
| − | padding-left: 19em;
| + | width: 100%; |
| − | padding-right: 1em;
| + | padding-left: 12em; |
| − | "><h6>Thermal image of the PCB</h6>
| + | padding-right: 1em; |
| − | </div>
| + | "><h6 style="text-align: center">Image 17. Hot and cold zones on the PCB and thermal image</h6> |
| | + | </div> |
| | </div> | | </div> |
| | + | </div> |
| | | | |
| | <div> | | <div> |
| | <ul> | | <ul> |
| | <li> | | <li> |
| − | <p> For the hot zone we are using a power resistor attached to the PCB from below and to control the current going through it we are using PWM. This means putting shortly quickly alternating between voltage on or off to create a pseudo analog voltage that is a percentage of the applied voltage depending on the duty cycle. If the pulse is longer the analog voltage would be closer to the real voltage.</p> | + | <p>For the <b>hot zone</b> we use a <b>power resistor</b> attached to the PCB from below and to control the current going through it we use PWM. This means putting shortly quickly alternating between voltage on or off to create a pseudo analog voltage that is a percentage of the applied voltage depending on the duty cycle. If the pulse is longer the analog voltage would be closer to the real voltage.</p> |
| | | | |
| | </li><li> | | </li><li> |
| − | <p> For the cold zone a Peltier effect device has been used. This effect consists of directly converting a difference in voltage to a difference in temperature due to the P and N semiconductors that, when carrying current, transfer the heat from one side to the other. For having a good precision and better efficiency we are using voltage control. Which means actually changing the DC voltage level to alter the temperature difference. With peltier effect devices PWM is not a suitable control method because they require constant current to properly function. </p> | + | <p>For the <b>cold zone</b> a <b>Peltier effect</b> device is being used. This effect consists of directly converting a difference in voltage into a difference in temperature due to the P and N semiconductors that, when carrying current, transfer the heat from one side to the other.</p> |
| | + | <p>This means that we have to get rid of the heat generated so we can keep the temperature difference that the peltiers produce. For that reason, we have implemented a <b>stock liquid cooling solution</b> from a computer that works by a liquid flowing constantly between the peltiers and a radiator, effectively dissipating the heat.</p> |
| | + | <div style=" |
| | + | padding-bottom: 1em; |
| | + | |
| | + | text-align: left; |
| | + | "> |
| | + | <a href="https://static.igem.org/mediawiki/2018/thumb/e/e3/T--Valencia_UPV--RefrigeradorUPV2018.jpeg/799px-T--Valencia_UPV--RefrigeradorUPV2018.jpeg" |
| | + | data-lightbox="true"> |
| | + | <img src="https://static.igem.org/mediawiki/2018/thumb/e/e3/T--Valencia_UPV--RefrigeradorUPV2018.jpeg/799px-T--Valencia_UPV--RefrigeradorUPV2018.jpeg" alt="" style=" |
| | + | margin-top: 0.5em; |
| | + | margin-bottom: 0.8em; |
| | + | border-radius: 0.3em; |
| | + | width:300px |
| | + | "> |
| | + | </a> |
| | + | <div style=" |
| | + | width: 100%; |
| | + | padding-left: 5em; |
| | + | padding-right: 1em; |
| | + | "><h6 style="text-align: left">Image 18. Liquid cooling device</h6> |
| | + | </div> |
| | + | </div> |
| | + | <p>For having a good precision and better efficiency we use voltage control. Which means actually changing the DC voltage level to alter the temperature difference. With peltier effect devices PWM is not a suitable control method because they require constant current to properly function. </p> |
| | </li> | | </li> |
| | | | |
| Line 1,045: |
Line 1,049: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>Resistance (left) and Peltier (right)</h6> | + | "><h6>Image 19. Resistance (left) and Peltier (right)</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 1,054: |
Line 1,058: |
| | | | |
| | | | |
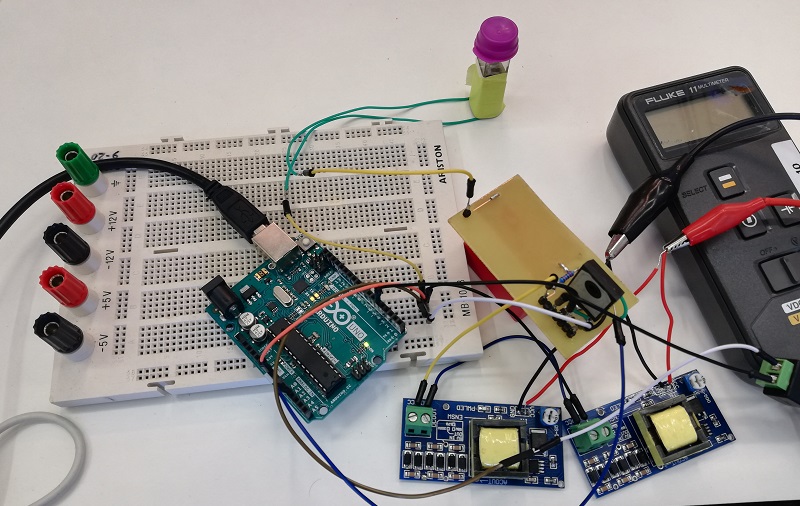
| − | <p> At both zones we are going to keep a thermistor monitoring the temperature to complete the control feedback loop. This thermistor is calibrated by using a lab grade thermometer and then adjusted using Steinhart-Hart model coefficients based on the readings. This means that we took readings on liquids with known temperatures and then read the raw resistor data. Based on those readings we can obtain the coefficients for our resistor-temperature curve and convert the voltage of a tension divider measured with our arduino into a really accurate temperature reading. </p> | + | <p> At both zones we are going to keep a <b>thermistor</b> monitoring the temperature to complete the control <b>feedback loop</b>. This thermistor is calibrated by using a lab grade thermometer and then adjusted using Steinhart-Hart model coefficients based on the readings. This means that we took readings on liquids with known temperatures and then read the raw resistor data. Based on those readings we can obtain the coefficients for our resistor-temperature curve and convert the voltage of a tension divider measured with our <b>arduino</b> into a really accurate temperature reading. </p> |
| | </div> | | </div> |
| | <a class="anchorOffset" id="electroporator"></a> | | <a class="anchorOffset" id="electroporator"></a> |
| Line 1,062: |
Line 1,066: |
| | | | |
| | <p> | | <p> |
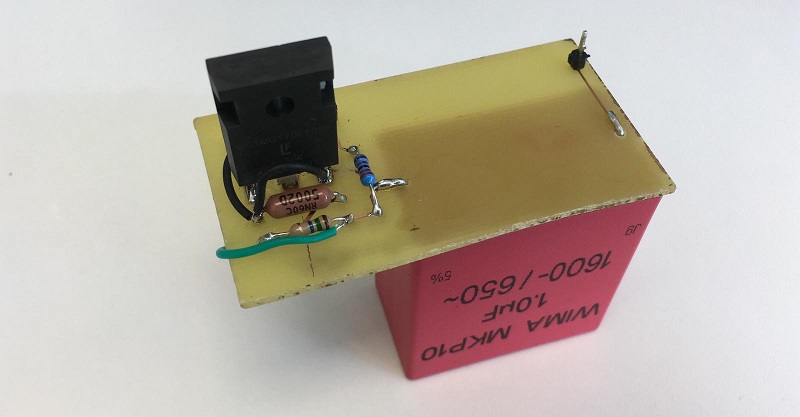
| − | We have chosen to do the transformation of the cells in Printeria by electroporation. The cells will enter Printeria via the entry carrousel in a pipette tip stored at -20ºC. We found that at this temperature the competent cells were transformed with sufficient efficiency for at least the first two weeks. </p> | + | We have chosen to do the transformation of the cells in Printeria by <b>electroporation</b>. The cells will enter Printeria via the entry carrousel in a pipette tip stored at -20ºC. We found that at this temperature the competent cells were transformed with sufficient efficiency for at least the first two weeks. </p> |
| | | | |
| − | <p> From this point on until the assembly reaction is complete, the competent cells are transported to a zone at 4ºC where they will remain stored. Once the assembly is complete the competent cells are mixed with the assembled genetic material and transported to the electroporator. | + | <p> From this point on until the assembly reaction is complete, the <b>competent cells</b> are transported to a zone at <b>4ºC</b> where they will remain <b>stored</b>. Once the assembly is complete the competent cells are mixed with the assembled genetic material and transported to the electroporator. |
| | | | |
| − | </p><p> The reason for choosing electroporation as a transformation method is that, as we have seen, it is the most efficient method outperforming heat shock and other commercial methods. Furthermore the implementation of the electroporator in Printeria was simple because the necessary electronic circuit had already been designed by the Valencia_UPV team in 2016 for the HYPE-IT project. </p> | + | </p><p> The reason for choosing electroporation as a transformation method is that, as we have seen, it is the <b>most efficient method</b> outperforming heat shock and other commercial methods. Furthermore, for the implementation of the electroporator in Printeria we have used the design developed by the <a href="https://2016.igem.org/Team:Valencia_UPV/Hardware" target="_blank">Valencia_UPV team in 2016 for the <b>HYPE-IT</b> project</a>, modifying its electronic circuit and designing a <b>new PCB</b>. </p> |
| | + | |
| | + | <div> |
| | + | <div class="row" style=" |
| | + | margin: 0; |
| | + | "> |
| | + | <div class="col-md-6" style=" |
| | + | padding: 0; |
| | + | padding-right: 0.3em; |
| | + | "> |
| | + | <a href="https://static.igem.org/mediawiki/2018/e/ed/T--Valencia_UPV--ELECTROPORADOR22UPV2018.jpeg" |
| | + | data-lightbox="true"> |
| | + | <img src="https://static.igem.org/mediawiki/2018/e/ed/T--Valencia_UPV--ELECTROPORADOR22UPV2018.jpeg" style=" |
| | + | margin-top: 0.5em;"> |
| | + | </a> |
| | + | </div> |
| | + | <div class="col-md-6" style=" |
| | + | padding: 0; |
| | + | padding-left: 0.3em; |
| | + | "> |
| | + | <a href="https://static.igem.org/mediawiki/2018/0/02/T--Valencia_UPV--ELECTROPORADOR23GIFUPV2018.jpeg" |
| | + | data-lightbox="true"> |
| | + | <img src="https://static.igem.org/mediawiki/2018/0/02/T--Valencia_UPV--ELECTROPORADOR23GIFUPV2018.jpeg" style=" |
| | + | margin-top: 0.5em; |
| | + | "> |
| | + | </a> |
| | + | </div> |
| | + | <div style=" |
| | + | width: 100%; |
| | + | padding-left: 19em; |
| | + | padding-right: 1em; |
| | + | "><h6>Image 20. Electroporator </h6> |
| | + | </div> |
| | + | </div> |
| | + | </div> |
| | + | |
| | + | <p>The electroporator is situated <b>on the tube</b> that transports the droplet with the DNA and bacteria to the <b>measurement zone</b>. The circuit charges a Capacitor at 1600V with step-up converters and then a simple circuit with a transistor is able to apply the voltage to the two metal plates placed on the transporting tube. This means that we can apply a voltage during a very short period of time, producing the required reaction.</p> |
| | | | |
| − | <p> As a summary, the circuit charges a Capacitor at 1600V with step-up converters. Then a simple circuit with a transistor is able to apply the voltage to the Cuvette. This means that we can apply a voltage during a very short period of time, producing the required reaction.</p>
| |
| | | | |
| | <div> | | <div> |
| Line 1,099: |
Line 1,138: |
| | padding-right: 1em; | | padding-right: 1em; |
| | padding-bottom: 1em; | | padding-bottom: 1em; |
| − | "><h6>Electroporator proof of concept</h6> | + | "><h6>Image 21. Electroporator proof of concept</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 1,320: |
Line 1,359: |
| | <h5>Measurement zone</h5> | | <h5>Measurement zone</h5> |
| | </div> | | </div> |
| − | <p> Also known as the output system, the measurement zone consists of an area separated from the rest of the zones in which the ideal conditions for the growth of bacteria are established. These conditions are achieved thanks to an electrical resistance that maintains the temperature of the whole zone at 37º and a shaker that keeps the test tubes moving to achieve a homogeneous mixture. In addition, sensors for OD and Fluorescence measurement have been implemented to monitor the entire process.</p> | + | <p> Also known as the output system, the measurement zone consists of an area <b>separated</b> from the rest of the zones in which the <b>ideal conditions</b> for the <b>growth of bacteria</b> are established. These conditions are achieved thanks to an electrical resistance that maintains the temperature of the whole zone at 37º and a <b>shaker</b> that keeps the test tubes moving to achieve a homogeneous mixture. In addition, sensors for <b>OD</b> and <b>fluorescence</b> measurement have been implemented to monitor the entire process.</p> |
| | | | |
| | <div style=" | | <div style=" |
| | padding-bottom: 1em; | | padding-bottom: 1em; |
| − | text-align: center; | + | text-align: left; |
| | "> | | "> |
| | <a href="https://static.igem.org/mediawiki/2018/d/d2/T--Valencia_UPV--Agitador3UPV2018.png" | | <a href="https://static.igem.org/mediawiki/2018/d/d2/T--Valencia_UPV--Agitador3UPV2018.png" |
| Line 1,338: |
Line 1,377: |
| | <div style=" | | <div style=" |
| | width: 100%; | | width: 100%; |
| − | padding-left: 7em; | + | padding-left: 3em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6 style="text-align: center">Output system 3D model</h6> | + | "><h6 style="text-align: left">Image 22. Output system 3D model</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 1,346: |
Line 1,385: |
| | | | |
| | | | |
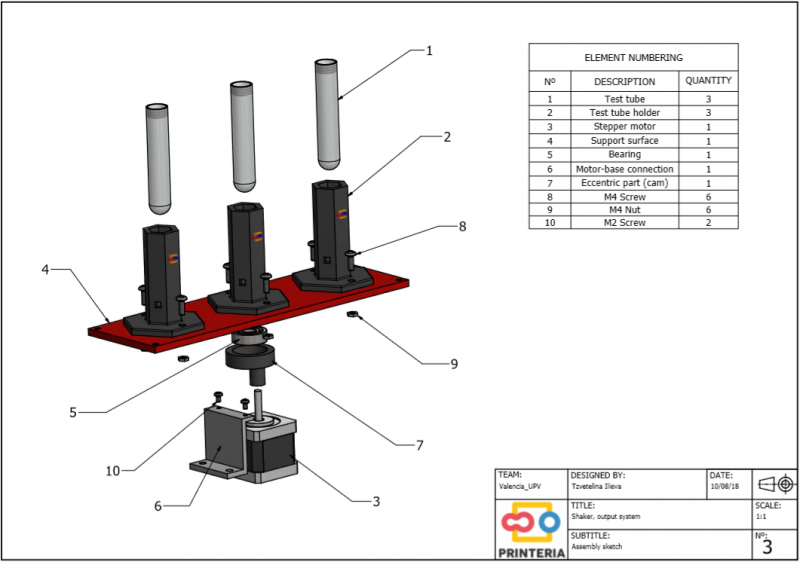
| − | <p> The shaker is achieved through the use of a stepper motor connected to a methacrylate base by means of an eccentric piece and a bearing. This eccentric piece produces the oscillating movement of the methacrylate base with the help of a spring that limits the rotating movement. </p> | + | <p> The shaker is achieved through the use of a <b>stepper motor</b> connected to a methacrylate base by means of an <b>eccentric piece</b> and a bearing. This eccentric piece produces the oscillating movement of the methacrylate base with the help of a spring that limits the rotating movement. </p> |
| | | | |
| | <div style=" | | <div style=" |
| Line 1,364: |
Line 1,403: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
| − | "><h6>Exploded view of the output system</h6> | + | "><h6>Image 23. Exploded view of the output system</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 1,400: |
Line 1,439: |
| | padding-left: 19em; | | padding-left: 19em; |
| | padding-right: 1em; | | padding-right: 1em; |
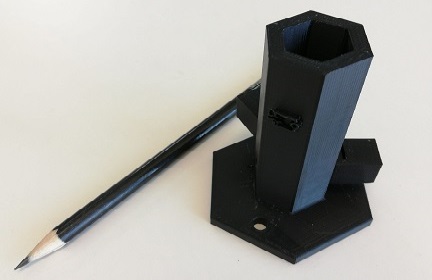
| − | "><h6>Eccentric piece and test tube's holder </h6> | + | "><h6>Image 24. Eccentric piece and test tube's holder </h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 1,407: |
Line 1,446: |
| | <h5 style="padding-left: 1em;">How does it work?</h5> | | <h5 style="padding-left: 1em;">How does it work?</h5> |
| | <div style="padding-left: 1em;"> | | <div style="padding-left: 1em;"> |
| − | <p>Before putting the machine into operation, the user must insert a 14 ml tube with 700ul of SOC (antibiotic-free recovery medium) into the output. Once the reactions have taken place in the experimentation area, the genetically modified and electroporated bacteria will be introduced into the 14ml tubes by means of peristaltic pumps where they will remain for 1 hour being shaken.</p> | + | <p>Before putting the machine into operation, the user must insert a 14 ml tube with <b>700ul of SOC</b> (antibiotic-free recovery medium) into the output. Once the reactions have taken place in the experimentation area, the genetically modified and <b>electroporated bacteria</b> will be introduced into the 14ml tubes by means of peristaltic pumps where they will remain for <b>1 hour</b> being shaken.</p> |
| − | <p>Subsequently, by means of the peristaltic pump, 3 ml of LB medium are introduced with the antibiotic to complete the bacterium growth cycle and after 16 hours the bacteria are ready and the user can remove them.</p> | + | <p>Subsequently, by means of the peristaltic pump, <b>3 ml of LB medium</b> are introduced with the <b>antibiotic</b> to complete the bacterium growth cycle and after <b>16 hours</b> the bacteria are ready and the user can remove them.</p> |
| | <p>It should be noted that although the number of bacteria transformed with plasmids without the correct assembly has been significantly reduced, the culture obtained will not be pure. In order to obtain a pure culture it would be necessary to always make a step by plate of the culture and to isolate a transformed colony with the correct assembly.</p> | | <p>It should be noted that although the number of bacteria transformed with plasmids without the correct assembly has been significantly reduced, the culture obtained will not be pure. In order to obtain a pure culture it would be necessary to always make a step by plate of the culture and to isolate a transformed colony with the correct assembly.</p> |
| | </div> | | </div> |
| Line 1,417: |
Line 1,456: |
| | <ul> | | <ul> |
| | <li> | | <li> |
| − | <p> <b> OD: </b> As for measuring the resulting bacteria we need a high precision light sensor we decided to use TSL235-LF. This converts the magnitude of light received into frequency, which can be precisely measured. Our sensor compares the light from a laser of 650 nm that passes through the experimentation tube over time. We chose a laser for a more stable light output. Comparing the values measured to the initial values without any bacteria we can calculate the percentage of absorbance of the medium, obtaining that way a suitable measurement of the cell population.</p> | + | <p> <b> OD: </b> As for measuring the resulting bacteria we need a high precision light sensor we decided to use <b>TSL235-LF</b>. This converts the magnitude of light received into frequency, which can be precisely measured. Our sensor compares the light from a <b>laser of 650 nm</b> that passes through the experimentation tube over time. We chose a laser for a more stable light output. Comparing the values measured to the initial values without any bacteria we can calculate the percentage of absorbance of the medium, obtaining that way a suitable <b>measurement</b> of the <b>cell population</b>.</p> |
| | + | <p> For the implementation of this sensor we took advantage of the design developed by the <a href="https://2016.igem.org/Team:Valencia_UPV/Hardware" target="_blank">Valencia_UPV team in 2016 for the HYPE-IT project</a> but we have enhanced the software that controls the OD meter.</p> |
| | </li> | | </li> |
| | <li> | | <li> |
| − | <p> <b> FL: </b>Similarly to our OD sensor we opted for the TSL235-LF for high precision light measuring. The only difference is that in this case the bacteria are excited with a LED of wavelength 480 nm, since it is the most suitable to excite them and obtain a fluorescent reaction from them. In front of the sensor we have put a filter that only allows light of 511 nm of wavelength to pass through. That way we obtain a result on the peak of fluorescent emission from the bacteria.</p> | + | <p> <b> Fluorescence: </b>Similarly to our OD sensor we opted for the <b>TSL235-LF</b> for high precision light measuring. The only difference is that in this case the bacteria are excited with a <b>LED</b> of wavelength <b>480 nm</b>, since it is the most suitable to excite them and obtain a fluorescent reaction from them. In front of the sensor we have put a <b>filter</b> that only allows light of <b>511 nm</b> of wavelength to pass through. That way we obtain a result on the peak of <b>fluorescent emission</b> from the bacteria.</p> |
| | </li> | | </li> |
| | </ul> | | </ul> |
| | + | <p> We have calibrated the data from the OD and Fl sensors with the OD and fluorescence data from the Biotek Cytation3 equipment obtaining both <a href="https://2018.igem.org/Team:Valencia_UPV/Demonstrate#curvesFOD" target="_blank">calibration curves</a>.</p> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 1,431: |
Line 1,472: |
| | padding-left: 1em; | | padding-left: 1em; |
| | "> | | "> |
| − | <a href="https://static.igem.org/mediawiki/2018/thumb/8/86/T--Valencia_UPV--AGITADORGIFUPV2018.jpeg/337px-T--Valencia_UPV--AGITADORGIFUPV2018.jpeg.gif" | + | <a href="https://static.igem.org/mediawiki/2018/8/86/T--Valencia_UPV--AGITADORGIFUPV2018.jpeg" |
| | data-lightbox="true"> | | data-lightbox="true"> |
| − | <img src="https://static.igem.org/mediawiki/2018/thumb/8/86/T--Valencia_UPV--AGITADORGIFUPV2018.jpeg/337px-T--Valencia_UPV--AGITADORGIFUPV2018.jpeg.gif" alt="" style=" | + | <img src="https://static.igem.org/mediawiki/2018/8/86/T--Valencia_UPV--AGITADORGIFUPV2018.jpeg" alt="" style=" |
| | margin-top: 0.5em; | | margin-top: 0.5em; |
| | margin-bottom: 0.8em; | | margin-bottom: 0.8em; |
| Line 1,443: |
Line 1,484: |
| | width: 100%; | | width: 100%; |
| | padding-right: 20em; | | padding-right: 20em; |
| − | "><h6>Shaker proof of concept</h6> | + | "><h6>Image 25. Shaker proof of concept</h6> |
| | </div> | | </div> |
| | </div> | | </div> |
| Line 1,545: |
Line 1,586: |
| | <h5>Disinfection and sterility</h5> | | <h5>Disinfection and sterility</h5> |
| | </div> | | </div> |
| − | <p> It is really important to keep a sterile atmosphere around the reaction. To ensure this we have implemented a series of measures to keep Printeria clean.</p> | + | <p> It is really important to keep a <b>sterile atmosphere</b> around the reaction. To ensure this we have implemented a series of measures to keep Printeria clean:</p> |
| | <div> | | <div> |
| | <ul> | | <ul> |
| | <li> | | <li> |
| − | <p> UltraViolet light: We have installed UV leds under the aluminum profiles in order to sterilize the reaction surface between uses. In order to achieve disinfection, the short wavelength of ultraviolet light is required to be between 280 and 100 nm.</p> | + | <p> UltraViolet light: We have installed <b>UV leds</b> under the aluminum profiles in order to sterilize the reaction surface between uses. In order to achieve disinfection, the short wavelength of ultraviolet light is required to be between <b>280 and 100 nm</b>.</p> |
| | </li> | | </li> |
| | <li> | | <li> |
| − | <p> An air filter with a 220 nm pore size on the peristaltic pump of the entrance.</p> | + | <p> An <b>air filter</b> with a <b>220 nm pore size</b> on the peristaltic pump of the entrance.</p> |
| | </li> | | </li> |
| | | | |
| Line 1,560: |
Line 1,601: |
| | | | |
| | <li> | | <li> |
| − | <p> Water and alcohol droplets that sweep across the surface in order to absorb any remaining of the reaction. | + | <p> <b>Water and alcohol or HCl droplets</b> that sweep across the surface in order to absorb any remaining of the reaction. |
| | </p> | | </p> |
| | </li> | | </li> |
| Line 1,566: |
Line 1,607: |
| | </ul> | | </ul> |
| | </div> | | </div> |
| − | <p>The disinfection process involves that once the test tube with the genetically modified bacteria has been removed, the user must place an empty tube in the exit position.</p> | + | <p>The <b>disinfection process</b> involves that once the test tube with the genetically modified bacteria has been removed, the user must place an empty tube in the exit position.</p> |
| − | <p>After that, by means of the entry system, drops of water and HCl will be introduced. These drops will go through all the pads to sweep all the genetic material that may have adhered to the surface and then will go to the extraction area of the PCB. Once there, they will be absorbed by the peristaltic pump and after going through the whole tube and cleaning it, they will fall into the test tube placed at the outlet. This tube will be removed by the user and placed in a autoclave bag for biological waste.</p> | + | <p>After that, by means of the entry system, <b>drops of water and HCl or alcohol</b> will be introduced. These drops will go through all the pads to sweep all the genetic material that may have adhered to the surface and then will go to the extraction area of the PCB. Once there, they will be absorbed by the <b>peristaltic pump</b> and after going through the whole tube and cleaning it, they will fall into the test tube placed at the outlet. This tube will be removed by the user and placed in a autoclave bag for biological waste.</p> |
| − | <p>Finally, after cleaning the surface, the UV leds will be activated and it will be completely disinfected and sterilized for a new use.</p> | + | <p>Finally, after cleaning the surface, the <b>UV leds</b> will be activated and it will be completely disinfected and sterilized for a new use.</p> |
| | <div> | | <div> |
| | <a class="anchorOffset" id="Simultaneous"></a> | | <a class="anchorOffset" id="Simultaneous"></a> |
| | <h5>Future of Printeria</h5> | | <h5>Future of Printeria</h5> |
| | </div> | | </div> |
| − | <p> One of the possible upgrades that we would do to Printeria for better usage in the future is the parallelization of experiments. This means being able to run multiple experiments at the same time in paralel. We support up to three reactions at the moment but we are limited to the design of the output zone and the input of the machine. We could alter this design to be able to do many more reactions at the same time, or one right after another to optimize the resources inside the machine.</p> | + | <p> One of the possible upgrades that we would do to Printeria for better usage in the future is the <b>parallelization of experiments</b>. This means being able to run multiple experiments at the same time in paralel. We support up to three reactions at the moment but we are limited by the design of the output zone and the input of the machine. We could alter this design to be able to do many more reactions at the same time, or one right after another to <b>optimize the resources</b> inside the machine.</p> |
| − | <p> In addition, one of the great advantages of Printeria is that the Output system can be independent from the rest. In this way, it is possible to introduce any culture of bacteria that has not been created in the machine to monitor its growth. Through the software, in a independent tab, it will be possible to only make measurements. The user will decide which measurements to make (OD, fluorescence...), and the results will be shown to the user and stored in his library of measurements, which could be accessed at any time</p> | + | <p> In addition, one of the great advantages of Printeria is that the <b>Output system</b> can be <b>independent</b> from the rest. In this way, it is possible to introduce any culture of bacteria that has not been created in the machine to <b>monitor its growth</b>. Through the software, in a independent tab, it will be possible to only make measurements. The user will decide which measurements to make (OD, fluorescence...), and the results will be shown and stored in his library of measurements, which could be accessed at any time.</p> |
| | | | |
| | </div> | | </div> |