
Improve
The part we decided to improve was part BBa_K133055, encoding an mCherry reporter gene. The improved protein is our mCherry, part BBa_K2810009, which has been codon optimised for use in Nicotiana bethamiana and has GoldenGate compatible 5' and 3' ends, containing the BsmBI/Esp3I recognition site, and DNA sequences that allow ligation into the plasmid pSB1C3, forming the BsaI recognition site in the process. This allows parts with compatible sticky ends following BsaI digestion to be ligated into a specific order. Below is a BLASTn result of the two sequences, where the query sequence is the original part, BBa_K133055, and the subject sequence is our improved part, BBa_K2810009.

Figure 1. BLAST alignment result showing the original sequence (query) and our new sequence (subject) showing nucleotides that have been changed.
We decided to improve an mCherry part as when using eGFP as a reporter gene we found that it had low expression, and inconsistent results when compared to our GUS reporter. We knew that eGFP functioned as a dimer, requiring more protein expression to get as much equivalent fluorescence as mCherry, which fluoresces as a monomer. eGFP also fluoresces in the green region of visible light. We found that eGFP expression was very similar to background levels, and we suspected this may be due to green wavelength photons being reflected from the leaf. However, several sources suggest that chlorophyll produces auto fluorescence in the red region of light, around 680nm(1). However, mCherry emission is measured at a lower wavelength, around 610nm, and so gave much clearer, less speculative fluorescence than eGFP. A comparison of eGFP and mCherry emission wavelengths is seen below, the original image from here. We obtained some convincing results and high quality characterisation with our promoters, 35S CaMV and RTBV, when using mCherry as the reporter gene.
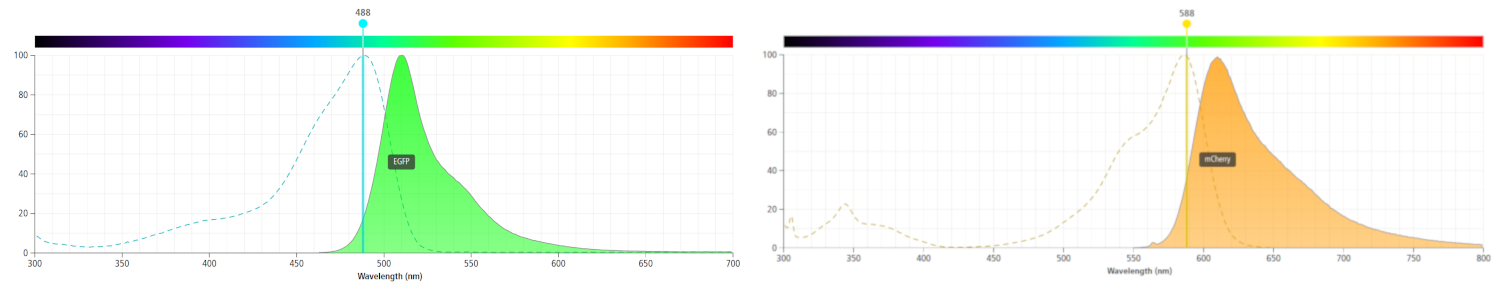
Figure 2. The emission wavelengths of eGFP (left) and mCherry (right) compared. It is likely that the small amounts background fluorescence picked up when measuring mCherry output are cholorphyll auto-fluorescence (at around 680nm). Nevertheless, the difference in gene expression is easily distinguishable.
Of course, as we were using GoldenGate cloning for our project, we could only use GoldenGate compatible parts to make our composite parts. This means that we were unable to experiment with the original parent part, BBa_k133055, as it is not GoldenGate compatible. For this reason, we only have results for our improved part, which, to our knowledge, is the only GoldenGate compatible mCherry reporter gene in the registry. All the tested constructs that used this improved part are shown below.

Figure 3. The collective results we obtained using mCherry, both in plants and bacteria.
References
(1) - Pedrós, R., Moya, I., Goulas, Y. and Jacquemoud, S. (2008). Chlorophyll fluorescence emission spectrum inside a leaf. Photochemical & Photobiological Sciences 7:498.










