Contents
Monomers
Abstract
An important step in the polymer industry is the production of the needed monomers. Their characteristics decide the properties of the final polymer. By modifying the metabolism of microorganisms, an environmentally friendly and sustainable way to produce glycolic acid, caprolactones and lactic acid can be established. These monomers are the basic components of the biodegradable polymers PLGA and PLGC.
Glycolic acid
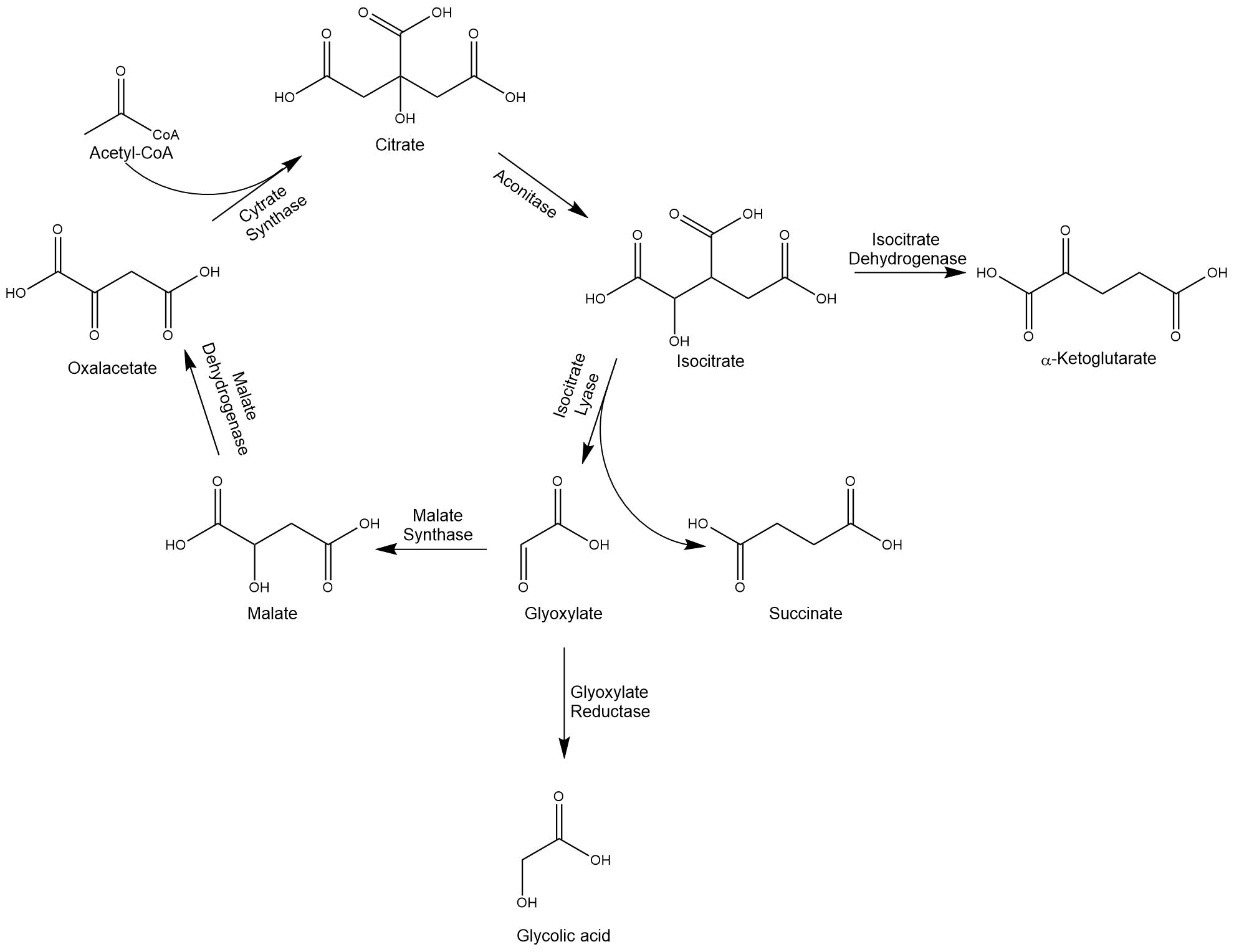
Glycolic acid is a simple α-hydroxy acid. Through the functional hydroxy- and acid-groups the molecule is highly soluble in water. This property makes glycolic acid polymers attractive for many applications in industry, e.g. in the textile, leather, oil, and gas industry[1]. The polymer of glycolic acid exhibits excellent gas barrier properties, which is an optimal base for e.g. packaging materials[2]. To enable the production of glycolic acid, we modified the glyoxylate cycle, which branches off the TCA cycle.
When acetyl-CoA yielding C2-units, e.g. acetate or ethanol, act as a sole carbon source for organisms such as E. coli and S. cerevisiae, the glyoxylate cycle functions as an anaplerotic pathway replenishing succinate.
The isocitrate lyase converts isocitrate into succinate and glyoxylate and thereby enables the bypassing of both decarboxylation steps of the TCA cycle. Another key enzyme is the malate synthase, which condenses glyoxylate with two acetyl-CoA molecules to isocitrate. The intermediate glyoxylate can also be reduced by a glyoxylate reductase to glycolic acid. [3] Because of the different characteristics of microorganisms, we decided to work with E. coli, as well as S. cerevisiae.
For more detailed information about the glycolic acid production in E. coli please look here. For the production in S. cerevisiae look here.
Caprolactone
ε-caprolactone is a seven-membered circular lactone. It is used as a building block for several polymers, like in our case poly(lactide-co-glycolide-co-caprolactone), abbreviated PLGC. The addition of ε-caprolactone to the polymer mix decreases its degradation time by making ester bonds easier accessible for water molecules. [1] Therefore, polymers with caprolactones have a shorter lifetime than polymers without them. Right now, ε-caprolactone is derived from petrochemicals and further chemicals, which are harmful for living beings and their environment. In order to produce ε-caprolactone in E. coli, we used two enzymes, cyclohexanone monooxygenase (CHMO) and alcohol dehydrogenase (ADH).
For more detailed information about the ε-caprolactone in E. coli please look here.
Lactic acid
In our project, we manufactured two co-polymers of polylactic-acid (PLA). For this, we genetically modified microorganisms to produce the monomers glycolic acid and ε-caprolactone. The monomer with the biggest mole fraction in these blends is lactic acid, which synthesis requires acetaldehyde, which is obtained from petro chemicials, and hydrogen cyanide, which is extremely toxic. Another, less toxic and less harmful to the environment, possibility to obtain the lactic acid is from microorganisms. We did not produce it on our own, as the Evry iGEM-Team did it 2016 successfully in Pseudomonas putida. To achieve that, they engineered its metabolic pathway. The parts they used and further details are shown on their page[4]. Poly-lactid-acid (PLA) is already a well-known biodegradable polymer and is used in several real-world applications, such as wrapping materials or suture stitches. Based on the results, of iGEM team Evry, we wanted to extend their idea of producing monomers biologically. We want to focus on the production of two other monomers that can be used for biological polymer production: glycolic acid and ε-caprolactone. With these three components, we aim to produce biodegradable co-polymers. The combination of biologically produced monomers and a biodegradable co-polymer brings the production of green polymers to the next level.
Model Organisms
Abstract
In our project we produced glycolic acid and caprolactones in E. coli and in S. cerevisiae. Both are common model organisms used in many laboratories and can be grown in relatively simple culture media. Their broad application is mainly attributed to their relative ease of genetic manipulation, allowing the expression of heterologous proteins.
Escherichia coli
E. coli bacteria are probably the most used bacteria in biotechnology. They contain a stable heterologous expression system and fast growth rates. Because this system has been used for genetic manipulation since 1973, it is well characterized. In addition to fast growth rates, e.g. compared to mammalian cells, it offers the ability to produce proteins in large quantities. Nowadays, it is widely utilized in the production of pharmaceuticals, enzymes, biofuels and fine chemicals. It has to be noted, that the production of high amounts of protein can lead to the formation of insoluble inclusion bodies which often have to be refolded before further use.
Saccharomyces cerevisiae
S. cerevisiae is a routinely used organism for the industrial production of fuels and chemicals, e.g. lactic acid, and for the production of consumables like bread and beer[5]. Genetic engineerings benefit from its ability to be genetically modified via homologous recombination, allowing the efficient introduction of constructs into the genome and gene knock-out or knock-downs. Furthermore, the ability to secrete folded protein attached to signal sequences, as well asits high tolerance towards low pH values make S. cerevisiae a suitable organism for the biotech industry. The recognized safe status[6] simplifies the approval of processes and products and reduces the problem of waste disposal.
Dimerization of organic acids
Abstract
Glycolide
Lactide
- ↑ Dischert et al., United States Patent Application Publication, Jul.12,2012[1].
- ↑ Engineering Escherichia coli for glycolic acid production from D-xylose through the Dahms pathway and glyoxylate bypass. [2].
- ↑ Stryer, Lubert(2002): Biochemistry, 5th Edition, New York: W H Freeman.
- ↑ Homepage Evry [3]
- ↑ Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation[4].
- ↑ Advances in metabolic engineering of yeast Saccharomyces cerevisiae for production of chemicals[5].